Karyomorphological Studies on Seven Variants of Clitoria Terantea L. (Fabaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Synopsis of Phaseoleae (Leguminosae, Papilionoideae) James Andrew Lackey Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1977 A synopsis of Phaseoleae (Leguminosae, Papilionoideae) James Andrew Lackey Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Botany Commons Recommended Citation Lackey, James Andrew, "A synopsis of Phaseoleae (Leguminosae, Papilionoideae) " (1977). Retrospective Theses and Dissertations. 5832. https://lib.dr.iastate.edu/rtd/5832 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

A Prespective Study of Clitoria Ternatia and Its Pharmacological Importance
High Technology Letters ISSN NO : 1006-6748 A PRESPECTIVE STUDY OF CLITORIA TERNATIA AND ITS PHARMACOLOGICAL IMPORTANCE S. GEJALAKSHMI *1, N. HARIKRISHNAN FACULTY OF PHARMACY, DR.M.G.R. EDUCATIONAL AND RESEARCH INSTITUTE, VELAPPANCHAVADI, CHENNAI-77 Abstract: Medicinal herbs and aromatic plants have been extensively used for the past few decades due to its potency and minimal side effects. By observing the medicinal importance of the climbing herb Clitoria ternatea (CT)of Fabeacea family and commonly known as Butterfly pea and Shankpushpi has been taken up due to its high medicinal value due to its wide range of use over decade as memory enhancer,antidepressant,anticonvulsant,transquilizers and sedative agent.A series of secondary metabolite including triterpenoids,flavone glycosides,anthocyanins and i steroids has been isolated from CT extracts.CT plant has wide range of pharmacological activity such as antimicrobial,antipyretic,diuretic,local anaesthetic.CT has been used for several diseases due to availability of several active constituents like alkaloids,flavanoids,saponins,tannins,carbohydrates .This review is an platform to explore the phytochemical investigation and pharmacological importance of CT,which have been practiced in traditional system of medicine and its future potential prespectives in view of innumerable therapeutic importance on this well-known twinning climber. Key words: Shankpushpi,phytochemical,antibacterial,anti- fungal, anti-cancer Introduction: Herbal drugs has an impact for curing disorders. The medicinal herbs are rich in various phytochemical constituents which has been found for traditional system of medicines. In the present reveiw focused on the traditional importance of clitoria ternatea. (CT).It is perennial twinning herb. It is a member of fabiaecea family and it has various synonym like blue pea. -

Butterfly Pea (Clitoria Ternatea) | Feedipedia
Butterfly pea (Clitoria ternatea) | Feedipedia Animal feed resources Feedipedia information system Home About Feedipedia Team Partners Get involved Contact us Butterfly pea (Clitoria ternatea) Automatic translation Description Nutritional aspects Nutritional tables References Sélectionner une langue ▼ Click on the "Nutritional aspects" tab for recommendations for ruminants, pigs, poultry, rabbits, horses, fish and crustaceans Feed categories All feeds Forage plants Cereal and grass forages Legume forages Forage trees Aquatic plants Common names Other forage plants Plant products/by-products Butterfly pea, blue pea, kordofan pea, cordofan pea, Asian pigeonwings [English]; pois bleu [French]; clitoria azul, azulejo, Cereal grains and by-products papito, zapatico de la reina, zapotillo, conchita azul, campanilla, bandera, choroque, lupita, pito de parra, bejuco de conchitas Legume seeds and by-products [Spanish]; cunhã, Fula criqua [Portuguese]; kittelbloem [Dutch]; Blaue Klitorie [German]; tembang telang [Indonesian]; Bunga Oil plants and by-products telang [Malay]; Mavi Kelebek Sarmaşığı [Turkish]; Chi Đậu biếc [Vietnamese]; [Bengali]; 蝶豆 [Chinese]; Fruits and by-products [Hindi]; [Malayalam]; [Marathi]; [Tamul]; [Telugu]; Roots, tubers and by-products ดอกอญชั นั [Thai] Sugar processing by-products Plant oils and fats Species Other plant by-products Feeds of animal origin Clitoria ternatea L. [Fabaceae] Animal by-products Dairy products/by-products Synonyms Animal fats and oils Insects Clitoria albiflora Mattei; Clitoria bracteata Poir.; Clitoria mearnsii De Wild.; Clitoria tanganicensis Micheli; Clitoria zanzibarensis Other feeds Vatke Minerals Other products Feed categories Legume forages Legume seeds and by-products Forage plants Latin names Plant and animal families Related feed(s) Plant and animal species Description Resources The butterfly pea (Clitoria ternatea L.) is a vigorous, trailing, scrambling or climbing tropical legume. -

Evolvulus Alsinoides (Convolvulaceae): an American Herb in the Old World Daniel F
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Available online at www.sciencedirect.com Journal of Ethnopharmacology 117 (2008) 185–198 Review Evolvulus alsinoides (Convolvulaceae): An American herb in the Old World Daniel F. Austin Arizona-Sonora Desert Museum, 2021 North Kinney Road, Tucson, AZ 85743, USA Received 23 October 2007; received in revised form 28 January 2008; accepted 29 January 2008 Available online 12 February 2008 Abstract People in the Indian region often apply shankhapushpi and vishnukranti, two Sanskrit-based common names, to Evolvulus alsinoides. These are pre-European names that are applied to a medicinal American species transported into the area. The period of introduction is uncertain, but probably took place in the 1500s or 1600s. Examination of relationships of Evolvulus alsinoides, geographic distribution, its names in Asia, medical uses, and chemical and laboratory analysis indicates that the alien plant was adopted, given an ancient Indian name, and incorporated into some Old World pharmacopoeias. The herb apparently was included in medicines because it not only reminded people of certain aspects of their gods and goddesses, but also because the chemicals it contained were useful against some maladies. -

Cultivar and Ecotype Recommendations for Partridge Pea
SC NRCS November 2015 Cultivar and Ecotype recommendations for Partridge Pea and Switchgrass (Guidance for CRP, CP-36) Problematic cultivar of Chamaecrista fasciculata (Large-Flower Partridge Pea)- "Lark" (AR) – not recommended This cultivar grows thick and tall, can cause longleaf pine seedling mortality and dominate wildlife habitat planting areas reducing diversity Comanche (TX) and Riley (KS) are other cultivars available commercially but because they originated outside of the southeast and are adapted for portions of Missouri, Arkansas, Tennessee, Mississippi, Louisiana, Oklahoma, and Texas; they are not recommended. Recommended/preferred alternatives: Large-Flower Partridge Pea (Chamaecrista fasciculata) - Florida ecotype or other Southeastern Regional ecotypes are available commercially - Seed vendors should provide seed ecotype information. Use light rate at 0.5 lb./acre or less. Use the closest ecotype available. If using large-flower partridge pea, do not seed until longleaf are several feet tall. Small-Flower Partridge Pea (Chamaecrista nictitans)- this species is smaller in stature and will not dominate or over-top longleaf seedlings. Slender Bushclover (Lespedeza virginica), Roundhead Lespedeza (Lespedeza capitata), Hairy Lespedeza (Lespedeza hirta); use 0.4 lbs. per acre or less Tick-trefoil/Beggar's Lice species: Desmodium canadense, D. floridanum, D. paniculatum, D. perplexum Wild Blue Lupine (Lupinus perennis), Goat's Rue (Tephrosia virginiana), or Butterfly Pea or Spurred Butterfly Pea (Clitoria mariana or Centrosema virginianum) Baptisia/Wild Indigo (Baptisia albescens, B. alba, B. australis, B. perfoliata, B. tinctoria) Sensitive Briar (Mimosa quadrivalvis or Mimosa microphylla) ← ↑ seeding rate for these: 0.1 to 0.5 lbs. per acre Problematic cultivars of Panicum virgatum (Switchgrass) – “Alamo” (TX), “Kanlow” (OK) – Not recommended - varieties were developed for forage and burn at the same BTU as a low grade coal). -

Healthy Food Traditions of Asia: Exploratory Case Studies From
Harmayani et al. Journal of Ethnic Foods (2019) 6:1 Journal of Ethnic Foods https://doi.org/10.1186/s42779-019-0002-x ORIGINALARTICLE Open Access Healthy food traditions of Asia: exploratory case studies from Indonesia, Thailand, Malaysia, and Nepal Eni Harmayani1, Anil Kumar Anal2, Santad Wichienchot3, Rajeev Bhat4, Murdijati Gardjito1, Umar Santoso1, Sunisa Siripongvutikorn5, Jindaporn Puripaatanavong6 and Unnikrishnan Payyappallimana7* Abstract Asia represents rich traditional dietary diversity. The rapid diet transition in the region is leading to a high prevalence of non-communicable diseases. The aim of this exploratory study was to document traditional foods and beverages and associated traditional knowledge that have potential positive health impacts, from selected countries in the region. The study also focused on identifying their importance in the prevention and management of lifestyle-related diseases and nutritional deficiencies as well as for the improvement of the overall health and wellbeing. This was conducted in selected locations in Indonesia, Thailand, Malaysia and Nepal through a qualitative method with a pre-tested documentation format. Through a detailed documentation of their health benefits, the study tries to highlight the significance of traditional foods in public health as well as their relevance to local market economies towards sustainable production and consumption and sustainable community livelihoods. Keywords: Traditional foods, Ethnic recipes, Asian health food traditions, Cultural dietary diversity, Indonesia, Thailand, Malaysia and Nepal Introduction Due to the dynamic adaptations to local biocultural con- Asia represents vast geographic, socioeconomic, bio- texts and refinement over generations through empirical logical, and cultural diversity. This is also reflected in the observations, they assume to have positive health impacts dietary diversity of traditional foods. -

Taking Plant Pharmaceuticals from Research to Production
From Farm to Pharm: Taking plant pharmaceuticals from research to production Benjamin Doffek BSc, MSc (Hons) 0000-0001-7583-426X A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2020 Institute for Molecular Bioscience Abstract Abstract Affordable production of pharmaceuticals with high potency and low side effects is a major challenge of the 21st century. Peptides are an emerging class of therapeutics that have the potential to marry the specificity and efficacy of protein drugs with the stability and membrane permeability of small molecule drugs. Although many peptides are amenable to chemical synthesis, their cost of production is high, as is the generation of waste products. Peptide production in plants has the potential to be a scalable, cost effective, and a less environmentally taxing alternative. Cyclotides, first discovered in Oldenlandia affinis, are a unique class of backbone cyclic peptides containing three stabilising disulfide bridges that form a knot-like structure. Their stability, and for some variants, the ability to traverse cellular membranes make them ideal candidates for pharmaceutical and agricultural applications. Even though highly constrained sterically, cyclotides are amenable to engineering by replacing native sequences with bioactive epitopes. In this thesis, cyclotide production strategies in plant cell suspensions are examined with a special focus placed on O. affinis as an archetypical cyclotide producer. Additional species investigated are Clitoria ternatea, Hybanthus enneaspermus, Nicotiana benthamiana, and Petunia hybrida. Insights into how cyclotides and their biosynthetic processing machinery are regulated in suspension plant cells are reported and provide first steps towards an affordable and environmentally friendly peptide production system in plants. -
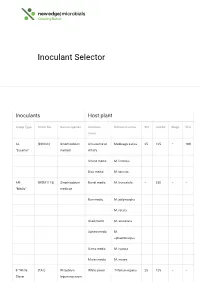
Inoculant Selector
Inoculant Selector Inoculants Host plant Group Type Strain No. Genus/species Common Botanical name Std Jumbo Mega Vial name AL (RRI128) Sinorhizobium All Lucerne or Medicago sativa 25 125 – 100 “Lucerne” meliloti Alfalfa Strand medic M. littoralis Disc medic M. tornata AM (WSM1115) Sinorhizobium Barrel medic M. truncatula – 250 – – “Medic” medicae Burr medic M. polymorpha M. rotata Snail medic M. scutellata Sphere medic M. sphaerocarpus Gama medic M. rugosa Murex medic M. murex B “White (TA1) Rhizobium White clover Trifolium repens 25 125 – – Clover leguminosarum bv. trifolii Red clover T. pratense Strawberry T. fragiferum clover Alsike clover T. hybridum Berseem, T. alexandrinum Egyptian clover Cluster, Ball T. glomeratum clover Suckling T. dubium clover C “Sub (WSM1325) Rhizobium Crimson Trifolium 50 250 – 200 Clover” leguminosarum clover incarnatum bv. trifolii Cupped clover T. cheleri Helmet clover T. clypeatum Rose clover T. hirtum Subterranean T. subterraneum clover var.subterraneum Subterranean T. subterraneum clover var.yanninicum Subterranean T. subterraneum clover var.brachy calycinum Arrowleaf T. vesiculosum 25 125 – 100 clover Balansa T. michelianum clover Bladder clover T. spumosum Gland clover T. glanduliferum Purple clover T. purpureum Persian clover T. resupinatum F “Faba & (WSM1455) Rhizobium Faba, Tick or Vicia faba – 500 1000 500 Pea” leguminosarum Broad bean bv. viciae Pea, Field pea Pisum sativum Common Vicia sativa – 500 1000 500 Vetch or Tare Bitter vetch V. ervilia Lathyrus Lathyruscicera Purple vetch V. benghalensis Woolly Pod V. villosa spp. vetch dasycarpa Lentil Lens culinaris – 250 500 250 G “Lupin” (WU425) Bradyrhizobium Narrow leaf Lupinus – 500 1000 500 sp. lupin angustifolius Mediterranean L. albus white lupin Yellow lupin L. -

Fruits and Seeds of Genera in the Subfamily Faboideae (Fabaceae)
Fruits and Seeds of United States Department of Genera in the Subfamily Agriculture Agricultural Faboideae (Fabaceae) Research Service Technical Bulletin Number 1890 Volume I December 2003 United States Department of Agriculture Fruits and Seeds of Agricultural Research Genera in the Subfamily Service Technical Bulletin Faboideae (Fabaceae) Number 1890 Volume I Joseph H. Kirkbride, Jr., Charles R. Gunn, and Anna L. Weitzman Fruits of A, Centrolobium paraense E.L.R. Tulasne. B, Laburnum anagyroides F.K. Medikus. C, Adesmia boronoides J.D. Hooker. D, Hippocrepis comosa, C. Linnaeus. E, Campylotropis macrocarpa (A.A. von Bunge) A. Rehder. F, Mucuna urens (C. Linnaeus) F.K. Medikus. G, Phaseolus polystachios (C. Linnaeus) N.L. Britton, E.E. Stern, & F. Poggenburg. H, Medicago orbicularis (C. Linnaeus) B. Bartalini. I, Riedeliella graciliflora H.A.T. Harms. J, Medicago arabica (C. Linnaeus) W. Hudson. Kirkbride is a research botanist, U.S. Department of Agriculture, Agricultural Research Service, Systematic Botany and Mycology Laboratory, BARC West Room 304, Building 011A, Beltsville, MD, 20705-2350 (email = [email protected]). Gunn is a botanist (retired) from Brevard, NC (email = [email protected]). Weitzman is a botanist with the Smithsonian Institution, Department of Botany, Washington, DC. Abstract Kirkbride, Joseph H., Jr., Charles R. Gunn, and Anna L radicle junction, Crotalarieae, cuticle, Cytiseae, Weitzman. 2003. Fruits and seeds of genera in the subfamily Dalbergieae, Daleeae, dehiscence, DELTA, Desmodieae, Faboideae (Fabaceae). U. S. Department of Agriculture, Dipteryxeae, distribution, embryo, embryonic axis, en- Technical Bulletin No. 1890, 1,212 pp. docarp, endosperm, epicarp, epicotyl, Euchresteae, Fabeae, fracture line, follicle, funiculus, Galegeae, Genisteae, Technical identification of fruits and seeds of the economi- gynophore, halo, Hedysareae, hilar groove, hilar groove cally important legume plant family (Fabaceae or lips, hilum, Hypocalypteae, hypocotyl, indehiscent, Leguminosae) is often required of U.S. -

U.S. EPA, Pesticide Product Label, HABITAT RELEASE 75SG
01/ ~'tI91 /8 BPA Reg_ u. s. Er.fV'IRONMENTAL PROTf>CTION AGBNCY Date of: 13Bua!lCC: Office of Pesticide Programs Number: Regiotration Division (7505C) JUL 29 ~ 401 RM· St., S.W. 241-402 mq Washington, D.C. 20460 Term of Issuance: NOTICE OF PESTICIDE: Conditional _x__ Registration Reregistration Name of Pesticide Product: (under FIFRA, as amended) Habitat Release 75SG herbicide Name and Address of Registrant (include ZIP Code) : American Cyanamid Company P.O. Box 400 Princeton, NJ 08543-0400 Not_: Changes in labeling differing in substance from that accepted in connection with this registration must be submitted to and accepted by the Registration Division prior to use of the label in commerce. In any correspondence on this product always refer to the above BpA registration number. On the basis ot information furnished by the registrant. the above named pesticide is hereby registered/reregistered ~der the Federal Ins~cticide, Fungicide and Rodenticide Act. Registration is in no way to be construed as ~ endorsement or recommendation of this product by the Agency.~ In order to protect health and the environment, the Administrator, on his motion, may at any time suspend or cancel the registration of a pesticide in accordance with the Act. The acceptance of any name in connection with the registration of a product under this Act is not to be construed as giving the registrant a right to exclusive use of the name or to its use if it has been covered by others. This product is conditionally registered in accordance with FIFRA sec. 3(c) (7) (A) provided that you: 1. -

35. ORCHIDACEAE/SCAPHYGLOTTIS 301 PSYGMORCHIS Dods
35. ORCHIDACEAE/SCAPHYGLOTTIS 301 PSYGMORCHIS Dods. & Dressl. each segment, usually only the uppermost persisting, linear, 5-25 cm long, 1.5-4.5 mm broad, obscurely emar- Psygmorchis pusilla (L.) Dods. & Dressl., Phytologia ginate at apex. Inflorescences single flowers or more com- 24:288. 1972 monly few-flowered fascicles or abbreviated, few-flowered Oncidium pusillum (L.) Reichb.f. racemes, borne at apex of stems; flowers white, 3.5-4.5 Dwarf epiphyte, to 8 cm tall; pseudobulbs lacking. Leaves mm long; sepals 3-4.5 mm long, 1-2 mm wide; petals as ± dense, spreading like a fan, equitant, ± linear, 2-6 cm long as sepals, 0.5-1 mm wide; lip 3.5-5 mm long, 2-3.5 long, to 1 cm wide. Inflorescences 1-6 from base of mm wide, entire or obscurely trilobate; column narrowly leaves, about equaling leaves, consisting of long scapes, winged. Fruits oblong-elliptic, ca 1 cm long (including the apices with several acute, strongly compressed, im- the long narrowly tapered base), ca 2 mm wide. Croat bricating sheaths; flowers produced in succession from 8079. axils of sheaths; flowers 2-2.5 cm long; sepals free, Common in the forest, usually high in trees. Flowers spreading, bright yellow, keeled and apiculate, the dorsal in the early dry season (December to March), especially sepal ca 5 mm long, nearly as wide, the lateral sepals in January and February. The fruits mature in the middle 4-5 mm long, 1-1.5 mm wide, hidden by lateral lobes to late dry season. of lip; petals to 8 mm long and 4 mm wide, bright yellow Confused with S. -

Centrosema Pascuorum Cv
African Journal of Biotechnology Vol. 11(92), pp. 15843-15851, 15 November, 2012 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB11.2412 ISSN 1684–5315 ©2012 Academic Journals Full Length Research Paper Variation and long term regenerative capacity of two important tropical forage legumes: Cavalcade (Centrosema pascuorum cv. Cavalcade) and Stylo 184 (Stylosanthes guianensis CIAT184) in vitro Varaporn Veraplakorn1, Malee Na Nakorn2, Lily Kaveeta2, Srisom Suwanwong2 and Ian James Bennett3* 1Department of Biotechnology, Faculty of Science, Ramkhamhaeng University, Bangkok 10240, Thailand. 2Department of Botany, Faculty of Science, Kasetsart University, Bangkok 10900, Thailand. 3School of Natural Sciences, Edith Cowan University, Western Australia. Accepted 27 July, 2012 Shoots of Cavalcade (Centrosema pascuorum cv. Cavalcade) and Stylo 184 (Stylosanthes guianensis CIAT 184) from in vitro germinated seeds were cultured on Murashige and Skoog (MS) medium supplemented with 0 to 7 mg L-1 N 6-benzyladenine (BA) in combination with 0 to 0.5 mg L-1 napthalene acetic acid (NAA) for shoot induction and MS supplemented with 0 to 0.5 mg L-1 indolebutyric acid (IBA) for root induction. For Cavalcade, the medium containing 1 mg L-1 BA produced the best shoot multiplication with an excess of six shoots produced from a single shoot (over four weeks) with a mean height 2.0 ± 0.01 cm. Adventitious shoot regeneration was obtained directly from stem axes. For Stylo 184, the maximum shoot regeneration (29.5 ± 1.0 cm shoots/explant) and height (1.5 ± 0.1 cm) was achieved using 7 mg L-1 BA and 0.01 mg L-1 NAA.