Antigen Presentation Damage Limitation: New Role for Immunoproteasomes
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Antigen Presentation by Dendritic Cells and Their Significance in Antineoplastic Immunotherapy
in vivo 18: 81-100 (2004) Antigen Presentation by Dendritic Cells and their Significance in Antineoplastic Immunotherapy BELA BODEY1,2, STUART E. SIEGEL1,2 and HANS E. KAISER3 1Department of Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA; 2Childrens’ Center for Cancer and Blood Diseases, Childrens’ Hospital Los Angeles, Los Angeles, CA; 3Department of Pathology, School of Medicine, University of Maryland, Baltimore, MD, U.S.A. and Department of General and Experimental Pathology, University of Vienna, Vienna, Austria Abstract. Dendritic cells (DCs) are present in essentially every derived peptides on the surface of major histocompatibility mammalian tissue, where they operate at the interface of innate complex (MHC) molecules and acquire the cellular specialization and acquired immunity by recognizing pathogens and presenting to select and activate naive antigen-specific T lymphocytes. pathogen-derived peptides to T lymphocytes. According to the Immunotherapeutic ideas are based on the ability of the research group of Shortman (1-9), experimental results suggest a mammalian immune system to recognize neoplastically "dual" DC differentiation model, demonstrating the existence of transformed cells. Immunotherapy of human neoplasms has both myeloid-derived (with characteristic IF: CD11b+, CD11c+, always represented a very attractive fourth-modality therapeutic CD8alpha- and DEC205+) and lymphoid-derived DCs (showing approach, especially in light of the many shortcomings of CD11b-, CD11c-, CD8alpha+ and DEC205+ IF). DCs, including conventional surgical, radiation and chemotherapies in the interdigitating cells (IDCs) and Langerhans cells (LCs), are management of neoplastically transformed cells. The cancer characterized by dendritic morphology, high migratory mobility and vaccine approach to therapy is based on the notion that the are the most effective, "professional" cells for antigen presentation immune system could possibly mount a rejection strength response in primary immune responses. -

Antigen Processing (Major Hbitocompatlbility Complex/Class I Moecules/Lymphokines) YOUNG YANG, JAMES B
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 4928-4932, June 1992 Immunology Proteasomes are regulated by interferon y: Implications for antigen processing (major hbitocompatlbility complex/class I moecules/lymphokines) YOUNG YANG, JAMES B. WATERS, KLAUS FROH, AND PER A. PETERSON Department of Immunology, The Scripps Research Institute, La Jolla, CA 92037 Communicated by Frank J. Dixon, February 27, 1992 ABSTRACT Class I major histocompatibility complex strengthened by the findings, presented in this communica- (MHC) molecules present antigenic peptides of cytoplasmic tion, that several proteasomal subunits, including MHC- origin to T cells. As the lengths ofthese peptides seem stried encoded subunits, are regulated by interferon y (IFN--y) and to eight or nine amino acids, an unusual proteolytic system that the incorporation of several more subunits into protea- must play a role in antigen processing. Proteasomes, a major somes appears to depend on the expression of the MHC- extralysosomal proteolytic system, are responsible for the encoded proteasomal subunits. Moreover, the pattern of degradation of cytoplasmic proteins. We demonstrate that expression of IFN-y-regulated subunits suggests complexi- several proteasomal subunits, including MHC-encoded sub- ties in the regulation of proteasomes with respect to its units, are regulated by interferon y. These data and the finding subunit composition, subcellular localization, and its incor- that MHC-encoded and other interferon -regulated protea- poration into larger ubiquitin-related proteolytic complexes. somal subunits are uniquely associated with proteasomes Possible functions for the MHC-encoded and IFN-y- strongly suggest that the immune system has recruited protea- regulated proteasomal subunits in antigen processing are somes for antigen processing. -

Cytoplasmic Proteolysis Antigens on MHC Class II Is Dependent On
Efficient Presentation of Both Cytosolic and Endogenous Transmembrane Protein Antigens on MHC Class II Is Dependent on Cytoplasmic Proteolysis This information is current as of September 28, 2021. Paushali Mukherjee, Aadish Dani, Sumeena Bhatia, Nagendra Singh, Alexander Y. Rudensky, Anna George, Vineeta Bal, Satyajit Mayor and Satyajit Rath J Immunol 2001; 167:2632-2641; ; doi: 10.4049/jimmunol.167.5.2632 Downloaded from http://www.jimmunol.org/content/167/5/2632 References This article cites 51 articles, 20 of which you can access for free at: http://www.jimmunol.org/content/167/5/2632.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 28, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2001 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Efficient Presentation of Both Cytosolic and Endogenous Transmembrane Protein Antigens on MHC Class II Is Dependent on Cytoplasmic Proteolysis1 Paushali Mukherjee,* Aadish Dani,† Sumeena Bhatia,* Nagendra Singh,* Alexander Y. -

On the Role of the Immunoproteasome in Transplant Rejection
Immunogenetics (2019) 71:263–271 https://doi.org/10.1007/s00251-018-1084-0 REVIEW On the role of the immunoproteasome in transplant rejection Michael Basler1,2 & Jun Li1,3 & Marcus Groettrup1,2 Received: 17 July 2018 /Accepted: 4 September 2018 /Published online: 15 September 2018 # Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract The immunoproteasome is expressed in cells of hematopoietic origin and is induced during inflammation by IFN-γ. Targeting the immunoproteasome with selective inhibitors has been shown to be therapeutically effective in pre-clinical models for autoim- mune diseases, colitis-associated cancer formation, and transplantation. Immunoproteasome inhibition prevents activation and proliferation of lymphocytes, lowers MHC class I cell surface expression, reduces the expression of cytokines of activated immune cells, and curtails T helper 1 and 17 cell differentiation. This might explain the in vivo efficacy of immunoproteasome inhibition in different pre-clinical disease models for autoimmunity, cancer, and transplantation. In this review, we summarize the effect of immunoproteasome inhibition in different animal models for transplantation. Keywords Proteasome . Immunoproteasome . Antigen processing . Antigen presentation . Transplantation Introduction et al. 2012). Depending on the cell type and the presence or absence of the pro-inflammatory cytokine interferon (IFN)-γ, The proteasome is responsible for the degradation of proteins the three inducible β subunits of the immunoproteasome low in the cytoplasm and nuclei of all eukaryotic cells and exerts molecular mass polypeptide (LMP)2 (β1i), multicatalytic en- numerous essential regulatory functions in nearly all cell bio- dopeptidase complex-like (MECL)-1 (β2i), and LMP7 (β5i), logical pathways. The 26S proteasome degrades poly- can, in addition to the corresponding constitutive subunits ubiquitylated protein substrates and consists of a 19S regulator β1c, β2c, and β5c, enrich the cellular assortment of catalyti- and a 20S proteolytic core complex. -
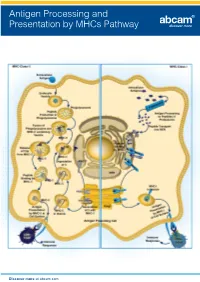
Antigen Processing and Presentation by Mhcs Pathway
Antigen Processing and Presentation by MHCs Pathway Discover more at abcam.com Antigen Processing and Presentation by MHCs Pathway Related antibodies Product This image shows MHC Class II highlights Product Clonality Applications Host Species Reactivity Product code expressing cells in formalin fixed and paraffin embedded human lymph MHC class I antibody [EP1395Y] M Flow Cyt, ICC/IF, IHC-P, IP, WB Rb Hu, Ms, Rat 52922 node, using ab55152. MHC class I antibody [AF6-88.5.5.3] (Biotin) M Flow Cyt Ms Ms 93528 MHC Class II antibody MHC class I antibody [34-1-2S] (FITC) M Flow Cyt Ms Ms 95572 (ab55152) MHC class I antibody [34-1-2S] (Phycoerythrin) M Flow Cyt Ms Ms 95571 MHC Class I alpha antibody [F21-2] M AP, Flow Cyt, IP, WB Ms Chk 23483 Clonality Applications MHC Class 1 H2 Db antibody [27-11-13] - BSA and Azide free M Flow Cyt, FuncS, IHC-Fr Ms Ms 25244 M IHC-P, WB MHC Class 1 H2 Db antibody [28-14-8] (FITC) M Flow Cyt Ms Ms 25056 Host Species cross reactivity MHC Class 1 H2 Db antibody [KH95] M Flow Cyt, FuncS, WB Ms Ms 64373 Ms Hu, RecFrag MHC Class I H2 Dd antibody [34-2-12] M Flow Cyt, FuncS, IHC-Fr, IP Ms Ms 64368 MHC Class I H2 Dk antibody [15-5-5.3] M Flow Cyt Ms Ms 25216 MHC Class II antigens are a valuable tool for studying T helper cell interactions with class II MHC Class I H2 Kb antibody P ELISA, WB Rb Ms 93364 positive antigen presenting cells, as well as the MHC Class I H2 Kd + Dd + q + u + v antibody [1.B.552] (FITC) M Flow Cyt, IP Ms Ms 62228 development of T helper cells since the antigen is present on stromal cells in the thymus. -

The Role of the Immunoproteasome in Interferon-Γ-Mediated Microglial Activation Received: 4 May 2017 Kasey E
www.nature.com/scientificreports OPEN The role of the immunoproteasome in interferon-γ-mediated microglial activation Received: 4 May 2017 Kasey E. Moritz1, Nikki M. McCormack1, Mahlet B. Abera2, Coralie Viollet3, Young J. Yauger1, Accepted: 14 July 2017 Gauthaman Sukumar3, Clifton L. Dalgard1,2,3,4 & Barrington G. Burnett 1,2 Published: xx xx xxxx Microglia regulate the brain microenvironment by sensing damage and neutralizing potentially harmful insults. Disruption of central nervous system (CNS) homeostasis results in transition of microglia to a reactive state characterized by morphological changes and production of cytokines to prevent further damage to CNS tissue. Immunoproteasome levels are elevated in activated microglia in models of stroke, infection and traumatic brain injury, though the exact role of the immunoproteasome in neuropathology remains poorly defned. Using gene expression analysis and native gel electrophoresis we characterize the expression and assembly of the immunoproteasome in microglia following interferon-gamma exposure. Transcriptome analysis suggests that the immunoproteasome regulates multiple features of microglial activation including nitric oxide production and phagocytosis. We show that inhibiting the immunoproteasome attenuates expression of pro-infammatory cytokines and suppresses interferon-gamma-dependent priming of microglia. These results imply that targeting immunoproteasome function following CNS injury may attenuate select microglial activity to improve the pathophysiology of neurodegenerative conditions or the progress of infammation-mediated secondary injury following neurotrauma. Microglia are the primary infammatory mediators of the central nervous system (CNS). Damage to the CNS results in the transition of microglia from a surveying or ‘ramifed’ state, to a ‘reactive’ state, allowing them to respond to changes in the local milieu1–3. -

Mechanisms of HIV Protein Degradation Into Epitopes: Implications for Vaccine Design
Viruses 2014, 6, 3271-3292; doi:10.3390/v6083271 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Mechanisms of HIV Protein Degradation into Epitopes: Implications for Vaccine Design Marijana Rucevic †, Julie Boucau †, Jens Dinter †, Georgio Kourjian † and Sylvie Le Gall * Ragon Institute of MGH, MIT and Harvard, Massachusetts General Hospital and Harvard Medical School, Cambridge, MA 02139, USA; E-Mails: [email protected] (M.R.); [email protected] (J.B.); [email protected] (J.D.); [email protected] (G.K.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-857-268-7010. Received: 11 June 2014; in revised form: 6 August 2014 / Accepted: 11 August 2014 / Published: 21 August 2014 Abstract: The degradation of HIV-derived proteins into epitopes displayed by MHC-I or MHC-II are the first events leading to the priming of HIV-specific immune responses and to the recognition of infected cells. Despite a wealth of information about peptidases involved in protein degradation, our knowledge of epitope presentation during HIV infection remains limited. Here we review current data on HIV protein degradation linking epitope production and immunodominance, viral evolution and impaired epitope presentation. We propose that an in-depth understanding of HIV antigen processing and presentation in relevant primary cells could be exploited to identify signatures leading to efficient or inefficient epitope presentation in HIV proteomes, and to improve the design of immunogens eliciting immune responses efficiently recognizing all infected cells. Keywords: HIV; antigen processing; protein degradation; proteasome; aminopeptidase; peptidase; immunogen; vaccine vector; dendritic cells; T cells; viral evolution Viruses 2014, 6 3272 1. -

Footprints of Antigen Processing Boost MHC Class II Natural Ligand
Barra et al. Genome Medicine (2018) 10:84 https://doi.org/10.1186/s13073-018-0594-6 RESEARCH Open Access Footprints of antigen processing boost MHC class II natural ligand predictions Carolina Barra1† , Bruno Alvarez1†, Sinu Paul2, Alessandro Sette2, Bjoern Peters2, Massimo Andreatta1, Søren Buus3 and Morten Nielsen1,4* Abstract Background: Major histocompatibility complex class II (MHC-II) molecules present peptide fragments to T cells for immune recognition. Current predictors for peptide to MHC-II binding are trained on binding affinity data, generated in vitro and therefore lacking information about antigen processing. Methods: We generate prediction models of peptide to MHC-II binding trained with naturally eluted ligands derived from mass spectrometry in addition to peptide binding affinity data sets. Results: We show that integrated prediction models incorporate identifiable rules of antigen processing. In fact, we observed detectable signals of protease cleavage at defined positions of the ligands. We also hypothesize a role of the length of the terminal ligand protrusions for trimming the peptide to the MHC presented ligand. Conclusions: The results of integrating binding affinity and eluted ligand data in a combined model demonstrate improved performance for the prediction of MHC-II ligands and T cell epitopes and foreshadow a new generation of improved peptide to MHC-II prediction tools accounting for the plurality of factors that determine natural presentation of antigens. Keywords: MHC-II, Binding predictions, Eluted ligands, T cell epitope, Neural networks, Antigen processing, Machine learning, Mass spectrometry Background MHC-II binding groove, unlike MHC class I, is open at Major histocompatibility complex class II (MHC-II) mole- both ends. -

Antigen Processing and Presentation Pamela Wearsch and Peter Cresswell
Antigen processing and presentation Pamela Wearsch and Peter Cresswell In antigen-presenting cells (APCs), such as dendritic cells (DCs) and B cells, heterogeneous intracellular phagocytosed by APCs, this simple division is not strictly enforced. Indeed, exogenous proteins pathways and mechanisms are responsible for generating complexes of MHC class I and class II internalized by DCs can generate peptide–MHC class I complexes that are recognized by CD8+ T cells, molecules with peptide antigens, and complexes of CD1 molecules with lipid antigens, for presentation a phenomenon referred to as cross-presentation. Similarly, endogenous and viral proteins can generate to T cells. This process — referred to as antigen processing and presentation — allows T cells to peptide–MHC class II complexes that are recognized by CD4+ T cells in a process involving autophagy. continuously assess the intracellular and extracellular milieu for signs of infection or abnormal cell Understanding the processes and mechanisms by which antigens are captured, processed and loaded growth. Although MHC class I molecules typically bind peptides derived from endogenous proteins onto MHC molecules for presentation to T cells provides us with crucial insights that are necessary for and MHC class II molecules typically bind peptides derived from proteins that are endocytosed or the design of vaccines and therapeutic strategies to bolster T-cell responses. The MHC class II pathway The MHC class I pathway MHC class II αβ-chain dimers are assembled in the endoplasmic reticulum (ER) All nucleated cells express MHC class I molecules and present endogenous peptide as a nonameric complex with the invariant chain (Ii), which protects against antigens to CD8+ T cells, but some DC subsets can also present exogenous premature peptide or protein interactions in pre-lysosomal peptides to CD8+ T cells through cross-presentation. -

The Role of the Immunoproteasome in Inflammatory Bowel Disease
The role of the immunoproteasome in inflammatory bowel disease vorgelegt von Dipl. Biochemikerin Nicole Schmidt zur Erlangung des akademischen Grades Doktorin der Naturwissenschaften (Dr. rer. nat.) von der Fakult¨atIII - Prozesswissenschaften der Technischen Universit¨atBerlin genehmigte Dissertation Promotionsausschuss: Vorsitzender: Prof. Dr. J. Kurreck Berichter: Prof. Dr. R. Lauster Berichter: PD Dr. U. Steinhoff Tag der wissenschaftlichen Aussprache: 16.04.2010 Berlin 2010 D83 1 2 Anybody who has been seriously engaged is scientific work of any kind realizes that over the entrance to the gates of the temple of science are written the words: 'Ye must have faith.' (Max Planck) 3 Content 1 Abstract 5 1.1 Deutsch . .5 1.2 English . .6 2 Introduction 7 2.1 The gastrointestinal immune system . .7 2.2 Inflammatory bowel disease . 10 2.2.1 Basis of inflammatory bowel disease . 10 2.2.2 Immune dysfunction in inflammatory bowel disease . 12 2.2.3 Treatment strategies for inflammatory bowel disease . 15 2.3 The proteasome system . 17 2.3.1 Function of the proteasome . 17 2.3.2 Proteasome structure . 19 2.4 The proteasome and inflammation . 22 2.4.1 Proteasome-mediated regulation of NF-κB.............. 22 2.4.2 The role of the proteasome in IBD . 26 2.4.3 Inhibitors of the proteasome . 27 2.5 The lmp7 knockout mouse . 29 2.6 Dextran sulfate sodium (DSS)-induced colitis model . 29 3 Aim of the study 31 4 Material and methods 32 4.1 Materials . 32 4.1.1 Antibodies . 32 4.2 Methods . 32 4.2.1 Mice . 32 4.2.2 Dextran sulfate sodium (DSS) induced colitis model . -

The Intermediate Proteasome Is Constitutively Expressed in Pancreatic Beta Cells And
bioRxiv preprint doi: https://doi.org/10.1101/753061; this version posted August 30, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 The intermediate proteasome is constitutively expressed in pancreatic beta cells and 2 upregulated by stimulatory, non-toxic concentrations of interleukin 1 3 4 Muhammad Saad Khilji1, Danielle Verstappen1,2, Tina Dahlby1, Michala Cecilie Burstein Prause3, 5 Celina Pihl1, Sophie Emilie Bresson1, Tenna Holgersen Bryde1, Kristian Klindt1, Dusan Zivkovic4, 6 Marie-Pierre Bousquet-Dubouch4, Björn Tyrberg5, Nils Billestrup3, Thomas Mandrup-Poulsen1, 7 Michal Tomasz Marzec1* 8 9 Affiliations 10 1 Laboratory of Immuno-endocrinology, Inflammation, Metabolism and Oxidation Section, 11 Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark 12 2 Radboud Universiteit, Nijmegen, Netherlands 13 3 Section for Beta-cell Biology, Department of Biomedical Sciences, University of Copenhagen, 14 Copenhagen, Denmark 15 4 Institut de Pharmacologie et de Biologie Structurale, Centre National de la Recherche 16 Scientifique, Université de Toulouse, 31077 Toulouse, France 17 5 Department of Physiology, Institute of Neuroscience and Physiology, Sahlgrenska Academy, 18 University of Gothenburg, Gothenburg, Sweden 19 20 Short title: The intermediate proteasome expression in pancreatic beta cells. 21 22 *Corresponding author 23 Associate Professor Michal Tomasz Marzec M.D., PhD. 24 Postal Address: Panum Institute 12.6.8, Blegdamsvej 3B, DK-2200 Copenhagen, Denmark 25 Phone: +45 25520256 26 Email: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/753061; this version posted August 30, 2019. -

Inhibition of the Immunoproteasome Subunit LMP7 with ONX&Nbsp
Biol Blood Marrow Transplant 21 (2015) 1555e1564 Biology of Blood and Marrow Transplantation journal homepage: www.bbmt.org Biology Inhibition of the Immunoproteasome Subunit LMP7 with ONX 0914 Ameliorates Graft-versus-Host Disease in an MHC-Matched Minor Histocompatibility AntigeneDisparate Murine Model Jenny Zilberberg 1,*, Jennifer Matos 1, Eugenia Dziopa 1, Leah Dziopa 1, Zheng Yang 1, Christopher J. Kirk 2, Shahin Assefnia 3, Robert Korngold 1 1 John Theurer Cancer Center, Hackensack University Medical Center, Hackensack, New Jersey 2 Onyx Pharmaceuticals, South San Francisco, California 3 Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC Article history: abstract Received 24 March 2015 In the current study we evaluated the effects of immunoproteasome inhibition using ONX 0914 (formerly Accepted 12 June 2015 PR-957) to ameliorate graft-versus-host disease (GVHD). ONX 0914, an LMP7-selective epoxyketone inhibitor of the immunoproteasome, has been shown to reduce cytokine production in activated monocytes and T cells Key Words: and attenuate disease progression in mouse models of rheumatoid arthritis, colitis, systemic lupus erythe- Immunoproteasome matosus, and, more recently, encephalomyelitis. Inhibition of LMP7 with ONX 0914 in the B10.BR/CBA MHC- GVHD matched/minor histocompatibility antigen (miHA)-disparate murine blood and marrow transplant (BMT) Murine models model caused a modest but significant improvement in the survival of mice experiencing GVHD. Concomitant BMT with these results, in vitro mixed lymphocyte cultures revealed that stimulator splenocytes, but not responder T cells, treated with ONX 0914 resulted in decreased IFN-g production by allogeneic T cells in both MHC-disparate (B10.BR anti-B6) and miHA-mismatched (B10.BR anti-CBA) settings.