Wine Traceability
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Beer VS Wine Dinner August 28Th 7Pm $110 Per Person All Inclusive
By the Glass White Red Giocato Pinot Grigio 2017 Slovenia $8 Altos de la Hoya Monastrell 2016 Spain $9 Von Der Leyen Riesling 2017 Germany $8 Gauchezco Res. Malbec 2016 Mendoza $10 Tormaresca Chardonnay 2017 Italy $9 Tasmorcan Barbera d’Asti 2016 Italy $11 Jelu Estate Torrontes 2016 Argentina $9 Treana Cabernet 2017 Paso Robles $12 Kennedy Point Sauvignon Blanc 2018 NZ $11 Fairfax Pinot Noir 2018 California $12 J. Bauer Gruner Veltliner 2016 Austria $11 Antiquum Juel Pinot Noir 2017 Willamette $20 Cowhorn Spiral 36 2017 Oregon $13 Mt Veeder Cabernet 2016 Napa Vly $20 Wind Racer Chardonnay 2015 RRV, CA $15 Quilceda Creek CVR 2013 Columbia Vly $25 Isole e Olena Cepparello 1999 Tuscany $25 Rosé Dessert/ Fortified Château De L’Escarelle 2018 Provence $9(Dry) Château du Cedre Sweet Malbec 2015 $8 Campus Oaks White Zin 2016 CA $7 El Maestro 15 Year Oloroso Sherry $8 (Dry) César Florida Moscatel Dorado Sherry $9 Taylor Fladgate Ruby Port $9 Sparkling Cevas Messias 10 Year Tawny Port $10 Paris-Simoneau Brut N/V France $8 Avantis Muscat Greece $10 Soligo Prosecco Extra Dry N/V Italy $10 Francois Voyer Pineau Des Charentes $11 Ramos Pinto LBV 2013 Port $12 Blandy’s 5 Year Malmsey Madeira $12 Broadbent Colheita 1996 Madeira $15 Spanish Wine Dinner Beer VS Wine Dinner July 24th 7pm August 28th 7pm $110 Per Person All Inclusive $110 Per Person All Inclusive “My only regret in life is that I did not drink more Legend has it that at The Wedding at Cana, there were wine”- Ernest Hemingway grumblings about there being no water transformed to beer. -

Smell Incredible in 2021 Hello Parfume Lovers!
THE ULTIMATE GUIDE SMELL INCREDIBLE IN 2021 HELLO PARFUME LOVERS! The beauty and true power of perfumes is that they are deeply personal. They can evoke strong memo- ries with a single note. They can draw us to others, creating special bonds. And they can make us feel exactly like we want to feel; sexy, beautiful, relaxed or bossy. In this way fragrance is also a form of ex- pression, a way of revealing your mood or personal- ity, giving others a piece of yourself simply through smell. In this little booklet, we share a few interesting, fun and practical facts and tips about all the awesome the ways fragrance can have an impact on your ev- eryday life. 2 CONTENTS FACTS YOU SHOULD KNOW ABOUT PERFUME 4 HOW TO CHOOSE »THE« FRAGRANCE? 8 FRAGRANCE STRUCTURE 11 FRAGRANCE FAMILIES 14 CHOOSE YOUR PERFECT »SECOND SKIN« FRAGRANCE 17 HOW DO YOU WANT TO FEEL? 23 THE POWER OF FRAGRANCE: HEALTH BENEFITS 25 FASCINATING LINK BETWEEN FRAGRANCES, EMOTIONS AND 28 BEHAVIOUR THE FORCE OF FRAGRANCE-ATTRACTION 30 PERFUME: THE STORY OF A MURDERER 33 THINGS NO ONE EVER TELLS YOU ABOUT PERFUME: 36 PERFUME: from hand-pressed to fully personalized 40 READY FOR THE FUTURE OF COSMETICS 43 FIRST SMART PERFUME IN THE WORLD 45 3 FACTS YOU SHOULD KNOW ABOUT PERFUME Fragrance and perfume are an important part of our everyday lives, yet we do not know a lot about their origin, background and frankly, simple day-to-day, usage-related facts. So, we’d like to take you on a brief perfume history tour and translate some of the commonly unknown phrases we often hear when shopping for our perfect perfume. -
Table of Contents
TABLE OF CONTENTS Sparkling & Champagne ............................. 3 White Wine .................................................. 4 Greece ........................................................................................4 Mediterranean ..................................................................... 6 Germany .................................................................................. 6 Italy ............................................................................................... 6 Spain ........................................................................................... 6 France ........................................................................................ 6 From the New World .......................................................7 Rosé Wine ................................................ 8 Skin-Contact Wine ................................... 9 Red Wine .................................................10 Greece .............................................................................10 Mediterranean ...........................................................13 Italy ..................................................................................... 13 Spain .................................................................................. 13 France................................................................................14 From the New World ............................................ 14 Thrace Macedonia Epirius Thessaly Ionian Islands Aegean Peloponnese Islands Crete 2 SPARKLING -

Wines by the Glass
WINES BY THE GLASS ROSÉ Macari Vineyards Estate Mattituck 2016 North Fork, NY 11/36 CHAMPAGNE & SPARKLING Segura Viudas Blanco Cava Brut NV Catalunya, SP 10 Btl 187ml Segura Viudas Rose Cava Brut NV Catalunya, SP 10 Btl 187ml Caviro Romio Prosecco NV Veneto, IT 10/36 I Borboni Asprinio Brut NV Campania, IT 49 Btl Ferrari Brut NV Trentino, IT 49 Ferrari Giulio Ferrari Brut Riserva del Fondatore 2002 Trentino, IT 270 Btl Champagne Philippe Gonet Brut Signature Blanc de Blancs NV Champagne, FR 40 Btl 375ml Champagne Fleury Brut Blancs de Noir Rose NV Biodynamic Champagne, FR 45 Btl 375ml La Caudrina Moscato d’Asti Piedmont, IT 10/34 500ml FROM THE TAP $9 per Glass Trebbiano/Poderi dal Nespoli/Sustainably Farmed 2016 Emilia-Romagna, IT Pinot Grigio/Venegazzu Montelvini Veneto, IT Chardonnay/Millbrook Estate/Sustainably Farmed 2014 Hudson River Valley, NY Barbera d’Alba/Cascina Pace/Sustainably Farmed 2015 Piedmont, IT Sangiovese/Poderi Dal Nespoli, Sangiovese Rubicone 2015 Emilia-Romagna, IT Merlot-Cabernet Sauvignon/Venegazzu Montelvini Veneto, IT WHITES BY THE GLASS/BOTTLE Cantina Cembra Sauvignon Blanc 2016 Trentino, IT 10/36 Figini Gavi di Gavi Cortese 2016 Piedmont, IT 12/40 Albino Armani “io” Pinot Grigio 2016 Trentino, IT 10/34 Paco y Lola Albarino 2013 Rias Baixas, SP 14/49 Suhru Riesling (Dry) 2016 Long Island, NY 11/39 Tenuta dell’ Ugolino Le Piaole “Castelli di Jesi” Verdicchio 2016 Marche, IT 10/38 Tenute Iuzzolini Ciro Bianco 2014 Calabria, IT 10/34 Marabino “Muscatedda” Moscato di Noto (Dry) 2014 Certified Organic Sicily, IT -

Our Namesake, Coda Di Volpe, Comes from a Grape Only Found in Southern Italy
WINE Our namesake, Coda di Volpe, comes from a grape only found in Southern Italy. Pulled from near extinction, it is one that expresses the true landscape & vineyards of Campania. Meaning “Tail of the Fox,” Coda di Volpe has influenced our entire wine program. Some of the most dynamic wines in the world are being made & bottled from the six traditional regions of Southern Italy; Campania, Basilicata, Puglia, Calabria, Sicily & Sardinia. Just as our namesake shows us a glimpse of the past, so do the other ancient varietals we have gathered on our list. By supporting small producers & native species, we strive to represent the vibrancy of Southern Italy’s present & future. We look forward to sharing our passion for those regions in every glass we pour. indicates native varietal once on the brink of extinction aperitivio wines Produced in the method of Fino Sherry & aged in chestnut barrels for a minimum of 10 years, Vernaccia di Oristano are complex & extremely rare. This ‘Italian Sherry’ has been made in Sardinia since the time of the Phoenicians Francesco Atzori Vernaccia di Oristano DOC 2006 $60 a multifaceted gem, meticulous winemaking translates to Vernaccia di Oristano DOC aromas of dried tangerine peel, tall grasses & marzipan, flavors glisten with sea spray, mint & chamomile- pair with cheeses & seafood for a reflective experience Francesco Atzori Vernaccia di Oristano DOC 1996 $60 hazelnut, dried marigold & polished mahogany unravel to Vernaccia di Oristano DOC reveal flavors of umami, tart pear & a saline, butterscotch finish. -
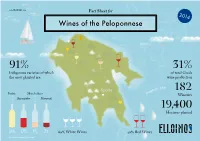
Wine Map of the Peleponnese 2014
www.ELLOINOS.com Fact Sheet for 2014 Wines of the Peloponnese Patras Athens 91% 31% Indigenous varieties of which of total Greek the most planted are: wine production n Sea Sparta gea 182 Ae Roditis Moschofilero Wineries Agiorgitiko Mavroudi 19,400 Hectares planted 34% 17% 9% 7% 60% White Wines 40% Red Wines Information design by ideologio Protected Designation of Origin Wine Colors Muscat of Rio Patras Grape: Muscat Blanc Mavrodaphne of Patras Grapes: Mavrodaphne, Korinthiaki Athens Nemea Grape Agiorgitiko Muscat of Patras Mantinia Grape: Muscat Blanc Grape Moschofilero Patras Epidaurus Grape: Roditis Sea ean eg Kalamata A In the EU, schemes of geographical indications known as Protected Designation of Origin (PDO) and Protected Monemvassia Geographical Indication (PGI), promote and protect names of —Malvasia quality agricultural and food products. Amongst many other products, the names of wines are also protected by these Grapes: Monemvassia (min 51%), laws. Assyrtiko, Asproudes, Kydonitsa PDO products are prepared, processed, and produced in a given geographical area, using recognized know-how and therefore acquire unique properties. White Wine Sweet White Wine Red Wine Sweet Red Wine Indigenous grapes International grapes Region Note There are additional grape varieties allowed, but PGI products are closely linked to the geographical current plantings are small. area in which they are traditionally and at least White indigenous: Asproudes Patras, Aidani, partially manufactured (prepared, processed OR Assyrtiko, Athiri, Glikerithra, Goustolidi, Laghorthi, produced), and have specific qualities attributable to Migdali, Petroulianos, Potamissi, Robola, Rokaniaris, Skiadopoulo, Sklava, Volitsa Aspro. that geographical area, therefore acquiring unique properties. Depending on their geographical breadth, Red indigenous: Limniona, Skylopnichtis, Thrapsa, Voidomatis, Volitsa. -

Langhe Doc Freisa
LANGHE D.O.C FREISA – BRUSAIA "Brusaia", or "burned": so is the land where one of the oldest vines of Piedmont grows, suitable both in clay soils than those marly and for this reason particularly common in the different wine areas of Piedmont. The Langhe Freisa presents ruby red COLOR with violet hues, that foretaste a complex SMELL, winey, fruity, typically even with some hints of vegetables. The TASTE is dry, very tannic and astringent: for its distin- ctive acid component, Freisa is perfect in PAIRING to elabo- rate meals, tasty and fat, such as those of traditional Piedmontese cuisine. ORGANOLEPTIC CHARACTERISTICS AND DESCRIPTION LOCATION OF THE VINEYARDS:: Piemonte - Langhe, Santo Stefano Belbo (CN) METHOD: The vinification of Freisa is the classic red vinifi- cation. The grapes are crushed, de-stemmed and fermented in vats at a controlled temperature (28/30 ° C) for about 7-10 days, depending on the vintage. In this period about 2/3 pum- pingover are made to promote fermentation and its processes. After fermentation, a transfer is made to eliminate the lees, then the wine is aged for about 3 months in stainless steel vats and subsequently put in bottles. COLOR: Dark ruby red, with purple tones. SMELL: Complex aroma, typically fruity, persistent and vinous with some hints of vegetal scents. TASTE: Dry, tannic and astringent. FOOD MATCHING: Once widely used because it matched very well with the old Piedmont cuisine. Because of its high acidity and tannins, it is great to be combined with high-fat meals as it leaves the palate very clean. -

Varietal Appellation Region Glera/Bianchetta
varietal appellation region 14 prairie organic vodka, fever tree ginger glera/bianchetta veneto, Italy 13 | 52 beer,cucumber, lime merlot/incrocio manzoni veneto, Italy 13 | 52 14 prairie organic gin, rhubarb, lime, basil 14 brooklyn gin, foro dry vermouth, melon de bourgogne 2016 loire, france 10 | 40 bigallet thym liqueur, lemon torrontes 2016 salta, argentina 10 | 40 14 albarino 2015 rias baixas, spain 12 | 48 foro amaro, cointreau, aperol, prosecco, orange dry riesling 2015 finger lakes, new york 13 | 52 14 pinot grigio 2016 alto-adige, italy 14 | 56 milagro tequila, jalapeno, lime, ancho chili salt chardonnay 2014 napa valley, california 14 | 56 14 sauvignon blanc 2015 loire, france 16 | 64 mount gay silver rum, beet, lime 14 calvados, bulleit bourbon, maple syrup, angostura bitters, orange bitters gamay 2016 loire, france 10 | 40 grenache/syrah 2016 provence, france 14 | 56 spain 8 cabernet/merlot 2014 bordeaux, france 10 | 40 malbec 2013 mendoza, argentina 13 | 52 illinois 8 grenache 2014 rhone, france 13 | 52 california 8 sangiovese 2014 tuscany, Italy 14 | 56 montreal 11 pinot noir 2013 central coast, california 16 | 64 massachusetts 10 cabernet sauvignon 2015 paso robles, california 16 | 64 Crafted to remove gluten pinot noir 2014 burgundy, france 17 | 68 massachusetts 7 new york 12 chardonnay/pinot noir NV new york 8 epernay, france 72 new york 9 pinot noir/chardonnay NV ay, france 90 england 10 2014 france 25 (750 ml) friulano 2011 friuli, Italy 38 gruner veltliner 2016 weinviertel, austria 40 france 30 (750 ml) sauvignon -

Le Colline Del Prosecco Di Conegliano E Valdobbiadene (Italy
Decision 42 COM 8B.31: Le Colline del Prosecco di Conegliano The World Heritage Committee, 1. Having examined Documents WHC/18/42.COM/8B, e Valdobbiadene WHC/18/42.COM/INF.8B1, and (Italy) WHC/18/42.COM/INF.8B4, 2. Refers the nomination of Le Colline del Prosecco di No 1571rev Conegliano e Valdobbiadene, Italy, back to the State Party, taking note of a potential of the proposed property to meet criteria (iv) and (v) to: 1. Redefine the nomination refocusing the potential Outstanding Universal Value on criteria (iv) and Official name as proposed by the State Party (v), Le Colline del Prosecco di Conegliano e Valdobbiadene 2. Redefine the boundaries and buffer zones of the nominated property, 3. Completing the adoption process by the 28 Location concerned municipalities of the tool “Technical Province of Treviso rule – Articolo Unico”, which was already approved Veneto Region by the Veneto Region in January 2018; Italy 3. Takes note that the general state of conservation of the site is adequate and that the adopted measures of Brief description conservation are generally effective, its monitoring and Located in the northern area of the Province of Treviso, in management systems are well-conceived and the Veneto Region, the Colline del Prosecco di structured, and the funding commitments by the relevant local authorities are to be saluted; Conegliano e Valdobbiadene comprises a portion of the 4. Commends the State Party for the structured governance vineyard landscape of Conegliano Valdobbiadene process to ensure cooperation among all public and Prosecco Superiore DOCG appellation wine production private actors involved in the site management as well as area. -
Retail Wine List
RETAIL WINE LIST Dear Friends, Guests & Wine Lovers: If you’re looking for a silver lining during these challenging times, well here it is! All wines on our extensive wine list are now available for takeout at retail prices! That means you save 50% on average, and a great bottle of wine makes a perfect pairing for your la Spiga meal at home. But, how to choose from so many?? Our awesome wine steward & sommelier, Dominic DeFilippo, is here to help! You may contact him in the following ways: T: 206.323.8881 (la Spiga)* C: 206.618.5667 (call or text)* E: [email protected] * Tues-Sat, 3pm to 6pm “anni e bicchieri d i vino non si contano ma i ” age and glasses of wine should never be counted Vini Frizzanti e Spumanti Ferrari Trento DOC Brut NV 28 Chardonnay Franciacorta 1701 Brut NV (Biodynamic, Organic) 30 Chardonnay, Pinot Nero, Lombardia Gianluca Viberti Casina Bric 460 Sparkling Rose Brut 27 Nebbiolo, Piemonte Contratto Millesimato Extra Brut ‘12 35 Pinot Nero, Chardonnay (Bottle Fermented, Natural Fermentation), Piedmont Palinieri “Sant’Agata” Lambrusco Sorbara ’18 17 Lambrusco Sorbara Quaresimo Lambrusco (Frizzante) NV 18 Lambrusco (Biodynamically farmed in Emilia Romagna!) Vini Spumanti Dolci (Sweet) Spinetta Moscato d’Asti (.375) 2017 16 Moscato Marenco Brachetto d’Acqui (.375) 2017 16 Brachetto Vini Bianchi ALTO ALDIGE Abazzia Novacella ‘17 22 Kerner Terlano Terlaner ‘17 30 Pinot Bianco, Sauvignon Blanc, Chardonnay Terlano Vorberg Riserva ‘17 46 Pinot Bianco “d ire pane al pane e vino al vino ” speak bread to bread and wine to wine -

Domini Veneti Corvina Merlot Raudii 2015
Lieber Fine Wines & Spirits 516-747-7989 Domini Veneti Corvina Merlot Raudii 2015 Product Info: Producer Name Cantina Valpolicella Negrar Product Name Domini Veneti Corvina Merlot Raudii Vintage 2015 Size 750ML / 6 Region Italy > Veneto > Veronese Classif/Style Igp Grape Corvina, Merlot Distributor Lieber Fine Wines & Spirits Item# #52671-15 Producer Since 1933, Cantina Valpolicella Negrar has told the story of men and women devoted to the crafting of quality wines: the finest, sincerest expression of a region home to more than 700 hectares of vineyards. Our grape-producers work with a smile, keen eye and dedication in a region that lingers in the heart. Cantina Valpolicella Negrar is historic cooperative winery with 230 members: families who work under the guidance of a highly qualified team as they live and breathe the poetry of a region that has been farmed with love for generations, the fruit of which becomes wine imbued with history and passion. Our work, however, is not merely growing grapes and making wine, as we have always conducted research alongside institutes and universities in a bid to use the latest technology to combine increasingly better quality with a lovingly crafted product. All the work in our vineyards is carried out by hand in order to leave the local environment and its biodiversity unharmed, continue the winemaking tradition of the Valpolicella Classica region, and be the driving force behind both social and cooperative projects. With great pride, generations of grape-producers have tended their vineyards and handed down the region's history and culture on behalf of Cantina Valpolicella Negrar. -

Wine Regulations 2005
S. I. of 2005 NATIONAL AGENCY FOR FOOD AND DRUG ADMINISTRATION AND CONTROL ACT 1993 (AS AMENDED) Wine Regulations 2005 Commencement: In exercise of the powers conferred on the Governing Council of the National Agency for Food and Drug Administration and Control (NAFDAC) by Sections 5 and 29 of the National Agency for Food and Drug Administration and Control Act 1993, as amended, and of all the powers enabling it in that behalf, THE GOVERNING COUNCIL OF THE NATIONAL AGENCY FOR FOOD AND DRUG ADMINISTRATION AND CONTROL with the approval of the Honourable Minister of Health hereby makes the following Regulations:- Prohibition: 1. No person shall manufacture, import, export, advertise, sell or distribute wine specified in Schedule I to these Regulations in Nigeria unless it has been registered in accordance with the provisions of these regulations. Use and Limit of 2. The use and limits of any food additives or food food additives. colours in the manufacture of wine shall be as approved by the Agency. Labelling. 3. (1) The labeling of wine shall be in accordance with the Pre-packaged Food (Labelling) Regulations 2005. (2) Notwithstanding Regulation 3 (i) of these Regulations, wines that contain less than 10 percent absolute alcohol by volume shall have the ‘Best Before’ date declared. 1 Name of Wine 4. (1) The name of every wine shall indicate the to indicate the accurate nature. nature etc. (2) Where a name has been established for the wine in these Regulations, such a name shall only be used. (3) Where no common name exists for the wine, an appropriate descriptive name shall be used.