Tropical Marine Invertebrates CAS BI 569
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Sexual Reproduction 7.2: Meiosis 7.3: Errors in Meiosis
Concepts of Biology Chapter 6 | Reproduction at the Cellular Level 135 6 | REPRODUCTION AT THE CELLULAR LEVEL Figure 6.1 A sea urchin begins life as a single cell that (a) divides to form two cells, visible by scanning electron microscopy. After four rounds of cell division, (b) there are 16 cells, as seen in this SEM image. After many rounds of cell division, the individual develops into a complex, multicellular organism, as seen in this (c) mature sea urchin. (credit a: modification of work by Evelyn Spiegel, Louisa Howard; credit b: modification of work by Evelyn Spiegel, Louisa Howard; credit c: modification of work by Marco Busdraghi; scale-bar data from Matt Russell) Chapter Outline 6.1: The Genome 6.2: The Cell Cycle 6.3: Cancer and the Cell Cycle 6.4: Prokaryotic Cell Division Introduction The individual sexually reproducing organism—including humans—begins life as a fertilized egg, or zygote. Trillions of cell divisions subsequently occur in a controlled manner to produce a complex, multicellular human. In other words, that original single cell was the ancestor of every other cell in the body. Once a human individual is fully grown, cell reproduction is still necessary to repair or regenerate tissues. For example, new blood and skin cells are constantly being produced. All multicellular organisms use cell division for growth, and in most cases, the maintenance and repair of cells and tissues. Single-celled organisms use cell division as their method of reproduction. 6.1 | The Genome By the end of this section, you will be able to: • Describe the prokaryotic and eukaryotic genome • Distinguish between chromosomes, genes, and traits The continuity of life from one cell to another has its foundation in the reproduction of cells by way of the cell cycle. -

The Evolution of Siphonophore Tentilla for Specialized Prey Capture in the Open Ocean
The evolution of siphonophore tentilla for specialized prey capture in the open ocean Alejandro Damian-Serranoa,1, Steven H. D. Haddockb,c, and Casey W. Dunna aDepartment of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06520; bResearch Division, Monterey Bay Aquarium Research Institute, Moss Landing, CA 95039; and cEcology and Evolutionary Biology, University of California, Santa Cruz, CA 95064 Edited by Jeremy B. C. Jackson, American Museum of Natural History, New York, NY, and approved December 11, 2020 (received for review April 7, 2020) Predator specialization has often been considered an evolutionary makes them an ideal system to study the relationships between “dead end” due to the constraints associated with the evolution of functional traits and prey specialization. Like a head of coral, a si- morphological and functional optimizations throughout the organ- phonophore is a colony bearing many feeding polyps (Fig. 1). Each ism. However, in some predators, these changes are localized in sep- feeding polyp has a single tentacle, which branches into a series of arate structures dedicated to prey capture. One of the most extreme tentilla. Like other cnidarians, siphonophores capture prey with cases of this modularity can be observed in siphonophores, a clade of nematocysts, harpoon-like stinging capsules borne within special- pelagic colonial cnidarians that use tentilla (tentacle side branches ized cells known as cnidocytes. Unlike the prey-capture apparatus of armed with nematocysts) exclusively for prey capture. Here we study most other cnidarians, siphonophore tentacles carry their cnidocytes how siphonophore specialists and generalists evolve, and what mor- in extremely complex and organized batteries (3), which are located phological changes are associated with these transitions. -

Xenacoelomorpha's Significance for Understanding Bilaterian Evolution
Available online at www.sciencedirect.com ScienceDirect Xenacoelomorpha’s significance for understanding bilaterian evolution Andreas Hejnol and Kevin Pang The Xenacoelomorpha, with its phylogenetic position as sister biology models are the fruitfly Drosophila melanogaster and group of the Nephrozoa (Protostomia + Deuterostomia), plays the nematode Caenorhabditis elegans, in which basic prin- a key-role in understanding the evolution of bilaterian cell types ciples of developmental processes have been studied in and organ systems. Current studies of the morphological and great detail. It might be because the field of evolutionary developmental diversity of this group allow us to trace the developmental biology — EvoDevo — has its origin in evolution of different organ systems within the group and to developmental biology and not evolutionary biology that reconstruct characters of the most recent common ancestor of species under investigation are often called ‘model spe- Xenacoelomorpha. The disparity of the clade shows that there cies’. Criteria for selected representative species are cannot be a single xenacoelomorph ‘model’ species and primarily the ease of access to collected material and strategic sampling is essential for understanding the evolution their ability to be cultivated in the lab [1]. In some cases, of major traits. With this strategy, fundamental insights into the a supposedly larger number of ancestral characters or a evolution of molecular mechanisms and their role in shaping dominant role in ecosystems have played an additional animal organ systems can be expected in the near future. role in selecting model species. These arguments were Address used to attract sufficient funding for genome sequencing Sars International Centre for Marine Molecular Biology, University of and developmental studies that are cost-intensive inves- Bergen, Thormøhlensgate 55, 5008 Bergen, Norway tigations. -

Feeding-Dependent Tentacle Development in the Sea Anemone Nematostella Vectensis ✉ Aissam Ikmi 1,2 , Petrus J
ARTICLE https://doi.org/10.1038/s41467-020-18133-0 OPEN Feeding-dependent tentacle development in the sea anemone Nematostella vectensis ✉ Aissam Ikmi 1,2 , Petrus J. Steenbergen1, Marie Anzo 1, Mason R. McMullen2,3, Anniek Stokkermans1, Lacey R. Ellington2 & Matthew C. Gibson2,4 In cnidarians, axial patterning is not restricted to embryogenesis but continues throughout a prolonged life history filled with unpredictable environmental changes. How this develop- 1234567890():,; mental capacity copes with fluctuations of food availability and whether it recapitulates embryonic mechanisms remain poorly understood. Here we utilize the tentacles of the sea anemone Nematostella vectensis as an experimental paradigm for developmental patterning across distinct life history stages. By analyzing over 1000 growing polyps, we find that tentacle progression is stereotyped and occurs in a feeding-dependent manner. Using a combination of genetic, cellular and molecular approaches, we demonstrate that the crosstalk between Target of Rapamycin (TOR) and Fibroblast growth factor receptor b (Fgfrb) signaling in ring muscles defines tentacle primordia in fed polyps. Interestingly, Fgfrb-dependent polarized growth is observed in polyp but not embryonic tentacle primordia. These findings show an unexpected plasticity of tentacle development, and link post-embryonic body patterning with food availability. 1 Developmental Biology Unit, European Molecular Biology Laboratory, 69117 Heidelberg, Germany. 2 Stowers Institute for Medical Research, Kansas City, MO 64110, -

Animal Phylum Poster Porifera
Phylum PORIFERA CNIDARIA PLATYHELMINTHES ANNELIDA MOLLUSCA ECHINODERMATA ARTHROPODA CHORDATA Hexactinellida -- glass (siliceous) Anthozoa -- corals and sea Turbellaria -- free-living or symbiotic Polychaetes -- segmented Gastopods -- snails and slugs Asteroidea -- starfish Trilobitomorpha -- tribolites (extinct) Urochordata -- tunicates Groups sponges anemones flatworms (Dugusia) bristleworms Bivalves -- clams, scallops, mussels Echinoidea -- sea urchins, sand Chelicerata Cephalochordata -- lancelets (organisms studied in detail in Demospongia -- spongin or Hydrazoa -- hydras, some corals Trematoda -- flukes (parasitic) Oligochaetes -- earthworms (Lumbricus) Cephalopods -- squid, octopus, dollars Arachnida -- spiders, scorpions Mixini -- hagfish siliceous sponges Xiphosura -- horseshoe crabs Bio1AL are underlined) Cubozoa -- box jellyfish, sea wasps Cestoda -- tapeworms (parasitic) Hirudinea -- leeches nautilus Holothuroidea -- sea cucumbers Petromyzontida -- lamprey Mandibulata Calcarea -- calcareous sponges Scyphozoa -- jellyfish, sea nettles Monogenea -- parasitic flatworms Polyplacophora -- chitons Ophiuroidea -- brittle stars Chondrichtyes -- sharks, skates Crustacea -- crustaceans (shrimp, crayfish Scleropongiae -- coralline or Crinoidea -- sea lily, feather stars Actinipterygia -- ray-finned fish tropical reef sponges Hexapoda -- insects (cockroach, fruit fly) Sarcopterygia -- lobed-finned fish Myriapoda Amphibia (frog, newt) Chilopoda -- centipedes Diplopoda -- millipedes Reptilia (snake, turtle) Aves (chicken, hummingbird) Mammalia -

Gfe Full Program 13.02.2019
Joint Meeting of the German and Israeli Societies of Developmental Biology Vienna, February 17-20, 2019 https://gfe2019.univie.ac.at/home/ Organizers Ulrich Technau, Eli Arama Co-Organizers Michael Brand, Fred Berger, Elly Tanaka, David Sprinzak, Peleg Hasson GfE https://www.vbio.de/gfe-entwicklungsbiologie IsSDB http://issdb.org Gesellschaft für Entwicklungsbiologie e.V. Geschäftsstelle: Dr. Thomas Thumberger Centre for Organismal Studies Universität Heidelberg Im Neuenheimer Feld 230 69120 Heidelberg E-mail: [email protected] Contents Sponsors ......................................................................................................................................... 4 General information ..................................................................................................................... 5 Venue .......................................................................................................................................... 5 Getting there................................................................................................................................ 5 From the airport ...................................................................................................................... 6 If you come by long distance train .......................................................................................... 6 Taxi ......................................................................................................................................... 6 If you -

Bodily Complexity: Integrated Multicellular Organizations for Contraction-Based Motility
fphys-10-01268 October 11, 2019 Time: 16:13 # 1 HYPOTHESIS AND THEORY published: 15 October 2019 doi: 10.3389/fphys.2019.01268 Bodily Complexity: Integrated Multicellular Organizations for Contraction-Based Motility Argyris Arnellos1,2* and Fred Keijzer3 1 IAS-Research Centre for Life, Mind & Society, Department of Logic and Philosophy of Science, University of the Basque Country, San Sebastián, Spain, 2 Department of Product and Systems Design Engineering, Complex Systems and Service Design Lab, University of the Aegean, Syros, Greece, 3 Department of Theoretical Philosophy, University of Groningen, Groningen, Netherlands Compared to other forms of multicellularity, the animal case is unique. Animals—barring some exceptions—consist of collections of cells that are connected and integrated to such an extent that these collectives act as unitary, large free-moving entities capable of sensing macroscopic properties and events. This animal configuration Edited by: Matteo Mossio, is so well-known that it is often taken as a natural one that ‘must’ have evolved, UMR8590 Institut d’Histoire et given environmental conditions that make large free-moving units ‘obviously’ adaptive. de Philosophie des Sciences et des Techniques (IHPST), France Here we question the seemingly evolutionary inevitableness of animals and introduce Reviewed by: a thesis of bodily complexity: The multicellular organization characteristic for typical Stuart A. Newman, animals requires the integration of a multitude of intrinsic bodily features between its New York Medical -

Carbonic Anhydrases: an Ancient Tool in Calcareous Sponge Biomineralization
fgene-12-624533 March 30, 2021 Time: 13:30 # 1 BRIEF RESEARCH REPORT published: 07 April 2021 doi: 10.3389/fgene.2021.624533 Carbonic Anhydrases: An Ancient Tool in Calcareous Sponge Biomineralization Oliver Voigt1*, Benedetta Fradusco1, Carolin Gut1, Charalampos Kevrekidis1, Sergio Vargas1 and Gert Wörheide1,2,3 1 Department of Earth and Environmental Sciences, Palaeontology and Geobiology, Ludwig-Maximilians-Universität München, Munich, Germany, 2 GeoBio-Center, Ludwig-Maximilians-Universität München, Munich, Germany, 3 SNSB-Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany Enzymes of the a-carbonic anhydrase gene family (CAs) are essential for the deposition of calcium carbonate biominerals. In calcareous sponges (phylum Porifera, class Calcarea), specific CAs are involved in the formation of calcite spicules, a unique trait and synapomorphy of this class. However, detailed studies on the CA repertoire of calcareous sponges exist for only two species of one of the two Calcarea subclasses, the Calcaronea. The CA repertoire of the second subclass, the Calcinea, has not been investigated so far, leaving a considerable gap in our knowledge about this Edited by: Melanie Debiais-Thibaud, gene family in Calcarea. Here, using transcriptomic analysis, phylogenetics, and in situ Université de Montpellier, France hybridization, we study the CA repertoire of four additional species of calcareous Reviewed by: sponges, including three from the previously unsampled subclass Calcinea. Our data Helena Cetkovi´ c,´ Rudjer Boskovic Institute, Croatia indicate that the last common ancestor of Calcarea had four ancestral CAs with defined Ana Riesgo, subcellular localizations and functions (mitochondrial/cytosolic, membrane-bound, and Natural History Museum, secreted non-catalytic). The evolution of membrane-bound and secreted CAs involved United Kingdom gene duplications and losses, whereas mitochondrial/cytosolic and non-catalytic CAs *Correspondence: Oliver Voigt are evidently orthologous genes. -

S I Section 4
3/31/2011 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Porifera Ecdysozoa Deuterostomia Lophotrochozoa Cnidaria and Ctenophora Cnidaria and Protostomia SSiection 4 Radiata Bilateria Professor Donald McFarlane Parazoa Eumetazoa Lecture 13 Invertebrates: Parazoa, Radiata, and Lophotrochozoa Ancestral colonial choanoflagellate 2 Traditional classification based on Parazoa – Phylum Porifera body plans Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Parazoa 4 main morphological and developmental Sponges features used Loosely organized and lack Porifera Ecdysozoa Cnidaria and Ctenophora tissues euterostomia photrochozoa D 1. o Presence or absence of different tissue L Multicellular with several types of types Protostomia cells 2. Type of body symmetry Radiata Bilateria 8,000 species, mostly marine Parazoa Eumetazoa 3. Presence or absence of a true body No apparent symmetry cavity Ancestral colonial Adults sessile, larvae free- choanoflagellate 4. Patterns of embryonic development swimming 3 4 1 3/31/2011 Water drawn through pores (ostia) into spongocoel Flows out through osculum Reproduce Choanocytes line spongocoel Sexually Most hermapppgggphrodites producing eggs and sperm Trap and eat small particles and plankton Gametes are derived from amoebocytes or Mesohyl between choanocytes and choanocytes epithelial cells Asexually Amoebocytes absorb food from choanocytes, Small fragment or bud may detach and form a new digest it, and carry -

Hazardous Marine Life
JOIN US AT DAN.ORG HAZARDOUS MARINE LIFE STINGS Cnidarians (nematocyst-carrying species) are HEALTH & DIVING REFERENCE SERIES responsible for more evenomations than any other marine phylum. These organisms contain stinging cells called cnidocyte that excel at venom delivery. 6 West Colony Place Durham, NC 27705 USA HAZARDOUS JELLYFISH PHONE: +1-919-684-2948 Of all the cnidarians, jellyfish cause the most MARINE LIFE: DAN EMERGENCY HOTLINE: +1-919-684-9111 frequent and severe human injuries. These result LEARN MORE AT DAN.ORG/HEALTH STINGS from direct contact with tentacles, and though painful, are not typically life threatening. PORTUGUESE MAN-OF-WAR Portuguese man-of-wars are free-floating cnidarians characterized by blue gas-filled bladders and long tentacles that drift on the ocean’s surface. Contact with a man-of-war’s Part #: 013-1040 Rev. 7.27.15 tentacles can cause significant pain and systemic symptoms. Their tentacles contain cnidocytes that deliver a potent proteic neurotoxin. Despite its resemblance to jellyfish, Portuguese man-of-war are more closely related to fire coral than to true jellyfish. HYDROIDS Although hydroids look like plants, these feathery cnidarians are actually a colony of small zooids that work together as a functioning animal. Like all cnidarians, these animals are armed with stinging cnidocytes. Hydroids, Portuguese man-of-war and fire coral belong to the same family, and contact with them can cause very similar reactions. Granted, their smaller contact area usually results in more localized and less dramatic reactions. FIRE CORAL Fire coral are colonial marine cnidarians that cause a mild to moderate burning reaction when touched. -

Basal Metazoans - Dirk Erpenbeck, Simion Paul, Michael Manuel, Paulyn Cartwright, Oliver Voigt and Gert Worheide
EVOLUTION OF PHYLOGENETIC TREE OF LIFE - Basal Metazoans - Dirk Erpenbeck, Simion Paul, Michael Manuel, Paulyn Cartwright, Oliver Voigt and Gert Worheide BASAL METAZOANS Dirk Erpenbeck Ludwig-Maximilians Universität München, Germany Simion Paul and Michaël Manuel Université Pierre et Marie Curie in Paris, France. Paulyn Cartwright University of Kansas USA. Oliver Voigt and Gert Wörheide Ludwig-Maximilians Universität München, Germany Keywords: Metazoa, Porifera, sponges, Placozoa, Cnidaria, anthozoans, jellyfishes, Ctenophora, comb jellies Contents 1. Introduction on ―Basal Metazoans‖ 2. Phylogenetic relationships among non-bilaterian Metazoa 3. Porifera (Sponges) 4. Placozoa 5. Ctenophora (Comb-jellies) 6. Cnidaria 7. Cultural impact and relevance to human welfare Glossary Bibliography Biographical Sketch Summary Basal metazoans comprise the four non-bilaterian animal phyla Porifera (sponges), Cnidaria (anthozoans and jellyfishes), Placozoa (Trichoplax) and Ctenophora (comb jellies). The phylogenetic position of these taxa in the animal tree is pivotal for our understanding of the last common metazoan ancestor and the character evolution all Metazoa,UNESCO-EOLSS but is much debated. Morphological, evolutionary, internal and external phylogenetic aspects of the four phyla are highlighted and discussed. SAMPLE CHAPTERS 1. Introduction on “Basal Metazoans” In many textbooks the term ―lower metazoans‖ still refers to an undefined assemblage of invertebrate phyla, whose phylogenetic relationships were rather undefined. This assemblage may contain both bilaterian and non-bilaterian taxa. Currently, ―Basal Metazoa‖ refers to non-bilaterian animals only, four phyla that lack obvious bilateral symmetry, Porifera, Placozoa, Cnidaria and Ctenophora. ©Encyclopedia of Life Support Systems (EOLSS) EVOLUTION OF PHYLOGENETIC TREE OF LIFE - Basal Metazoans - Dirk Erpenbeck, Simion Paul, Michael Manuel, Paulyn Cartwright, Oliver Voigt and Gert Worheide These four phyla have classically been known as ―diploblastic‖ Metazoa. -
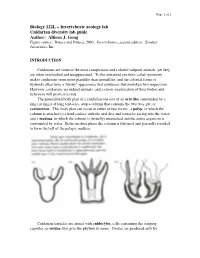
Cnidarians Are Some of the Most Conspicuous and Colorful Tidepool Animals, Yet They Are Often Overlooked and Unappreciated
Page 1 of 1 Biology 122L – Invertebrate zoology lab Cnidarian diversity lab guide Author: Allison J. Gong Figure source: Brusca and Brusca, 2003. Invertebrates, second edition. Sinauer Associates, Inc. INTRODUCTION Cnidarians are some of the most conspicuous and colorful tidepool animals, yet they are often overlooked and unappreciated. To the untrained eye their radial symmetry makes cnidarians seem more plantlike than animallike, and the colonial forms of hydroids often have a "bushy" appearance that reinforces that mistaken first impression. However, cnidarians are indeed animals, and a closer examination of their bodies and behaviors will prove it to you. The generalized body plan of a cnidarian consists of an oral disc surrounded by a ring (or rings) of long tentacles, atop a column that contains the two-way gut, or coelenteron. This body plan can occur in either of two forms: a polyp, in which the column is attached to a hard surface with the oral disc and tentacles facing into the water; and a medusa, in which the column is (usually) unattached and the entire organism is surrounded by water. In the medusa phase the column is flattened and generally rounded to form the bell of the pelagic medusa. Cnidarian tentacles are armed with cnidocytes, cells containing the stinging capsules, or cnidae, that give the phylum its name. Cnidae are produced only by Page 2 of 2 cnidarians, although they can occasionally be found in the cerata of nudibranch molluscs that feed on cnidarians: through a process that is not understood, some nudibranchs are able to ingest cnidae from their prey and sequester them, unfired, in their cerata for defense against their own predators.