Xenacoelomorpha's Significance for Understanding Bilaterian Evolution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Assembly of the Cnidarian Camera-Type Eye from Vertebrate-Like Components
Assembly of the cnidarian camera-type eye from vertebrate-like components Zbynek Kozmik*†, Jana Ruzickova*‡, Kristyna Jonasova*‡, Yoshifumi Matsumoto§, Pavel Vopalensky*, Iryna Kozmikova*, Hynek Strnad*, Shoji Kawamura§, Joram Piatigorsky†¶, Vaclav Paces*, and Cestmir Vlcek*† *Institute of Molecular Genetics, Academy of Sciences of the Czech Republic, Videnska 1083, 142 20 Prague 4, Czech Republic; §Graduate School of Frontier Sciences, University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8562, Japan; and ¶National Eye Institute, National Institutes of Health, Bethesda, MD 20892-3655 Edited by Eviatar Nevo, University of Haifa, Haifa, Israel, and approved April 11, 2008 (received for review January 14, 2008) Animal eyes are morphologically diverse. Their assembly, however, containing Tripedalia eyes have sophisticated visual optics as do always relies on the same basic principle, i.e., photoreceptors advanced bilaterian phyla (11). located in the vicinity of dark shielding pigment. Cnidaria as the In the present work, we characterize genes required for the likely sister group to the Bilateria are the earliest branching phylum assembly of camera-type eyes in Tripedalia. We show that the with a well developed visual system. Here, we show that camera- genetic building blocks typical of vertebrate eyes, namely ciliary type eyes of the cubozoan jellyfish, Tripedalia cystophora, use opsin and the melanogenic pathway, are used by the cubozoan genetic building blocks typical of vertebrate eyes, namely, a ciliary eyes. Although our findings of unsuspected parallelism are phototransduction cascade and melanogenic pathway. Our find- consistent with either an independent origin or common ances- ings indicative of parallelism provide an insight into eye evolution. try of cubozoan and vertebrate eyes, we believe the present data Combined, the available data favor the possibility that vertebrate favor the former alternative. -

1 Eric Davidson and Deep Time Douglas H. Erwin Department Of
Eric Davidson and Deep Time Douglas H. Erwin Department of Paleobiology, MRC-121 National Museum of Natural History Washington, DC 20013-7012 E-mail: [email protected] Abstract Eric Davidson had a deep and abiding interest in the role developmental mechanisms played in the generating evolutionary patterns documented in deep time, from the origin of the euechinoids to the processes responsible for the morphological architectures of major animal clades. Although not an evolutionary biologist, Davidson’s interests long preceded the current excitement over comparative evolutionary developmental biology. Here I discuss three aspects at the intersection between his research and evolutionary patterns in deep time: First, understanding the mechanisms of body plan formation, particularly those associated with the early diversification of major metazoan clades. Second, a critique of early claims about ancestral metazoans based on the discoveries of highly conserved genes across bilaterian animals. Third, Davidson’s own involvement in paleontology through a collaborative study of the fossil embryos from the Ediacaran Doushantuo Formation in south China. Keywords Eric Davidson – Evolution – Gene regulatory networks – Bodyplan – Cambrian Radiation – Echinoderms 1 Introduction Eric Davidson was a developmental biologist, not an evolutionary biologist or paleobiologist. He was driven to understand the mechanisms of gene regulatory control and how they controlled development, but this focus was deeply embedded within concerns about the relationship between development and evolution. Questions about the origin of major metazoan architectures or body plans were central to Eric’s concerns since at least the late 1960s. His 1971 paper with Roy Britten includes a section on “The Evolutionary Growth of the Genome” illustrated with a figure depicting variations in genome size in major animal groups and a metazoan phylogeny (Britten and Davidson 1971). -

A Phylum-Wide Survey Reveals Multiple Independent Gains of Head Regeneration Ability in Nemertea
bioRxiv preprint doi: https://doi.org/10.1101/439497; this version posted October 11, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. A phylum-wide survey reveals multiple independent gains of head regeneration ability in Nemertea Eduardo E. Zattara1,2,5, Fernando A. Fernández-Álvarez3, Terra C. Hiebert4, Alexandra E. Bely2 and Jon L. Norenburg1 1 Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA 2 Department of Biology, University of Maryland, College Park, MD, USA 3 Institut de Ciències del Mar, Consejo Superior de Investigaciones Científicas, Barcelona, Spain 4 Institute of Ecology and Evolution, University of Oregon, Eugene, OR, USA 5 INIBIOMA, Consejo Nacional de Investigaciones Científicas y Tecnológicas, Bariloche, RN, Argentina Corresponding author: E.E. Zattara, [email protected] Abstract Animals vary widely in their ability to regenerate, suggesting that regenerative abilities have a rich evolutionary history. However, our understanding of this history remains limited because regeneration ability has only been evaluated in a tiny fraction of species. Available comparative regeneration studies have identified losses of regenerative ability, yet clear documentation of gains is lacking. We surveyed regenerative ability in 34 species spanning the phylum Nemertea, assessing the ability to regenerate heads and tails either through our own experiments or from literature reports. Our sampling included representatives of the 10 most diverse families and all three orders comprising this phylum. -

Zootaxa, Platyhelminthes, Acoela, Acoelomorpha, Convolutidae
Zootaxa 1008: 1–11 (2005) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 1008 Copyright © 2005 Magnolia Press ISSN 1175-5334 (online edition) Waminoa brickneri n. sp. (Acoela: Acoelomorpha) associated with corals in the Red Sea MAXINA V. OGUNLANA1, MATTHEW D. HOOGE1,4, YONAS I. TEKLE3, YEHUDA BENAYAHU2, ORIT BARNEAH2 & SETH TYLER1 1Department of Biological sciences, The University of Maine, 5751 Murray Hall, Orono, ME 04469-5751, USA., e-mail: [email protected], [email protected], [email protected] 2 Department of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Ramat Aviv, Tel Aviv, 69978, Israel., e-mail: [email protected] 3 Department of Systematic Zoology, Evolutionary Biology Centre, Upsala University, Norbyvägen 18D, SE- 752 36 Uppsala, Sweden., e-Mail: [email protected] 4 Author of correspondence Abstract While the majority of acoels live in marine sediments, some, usually identified as Waminoa sp., have been found associated with corals, living closely appressed to their external surfaces. We describe a new species collected from the stony coral Plesiastrea laxa in the Red Sea. Waminoa brickneri n. sp. can infest corals in high numbers, often forming clusters in non-overlapping arrays. It is bronze-colored, owing to the presence of two types of dinoflagellate endosymbionts, and speckled white with small scattered pigment spots. Its body is disc-shaped, highly flattened and cir- cular in profile except for a small notch at the posterior margin where the reproductive organs lie. The male copulatory organ is poorly differentiated, but comprises a seminal vesicle weakly walled by concentrically layered muscles, and a small penis papilla with serous glands at its juncture with the male pore. -

A Phylogenetic Analysis of Myosin Heavy Chain Type II Sequences Corroborates That Acoela and Nemertodermatida Are Basal Bilaterians
A phylogenetic analysis of myosin heavy chain type II sequences corroborates that Acoela and Nemertodermatida are basal bilaterians I. Ruiz-Trillo*, J. Paps*, M. Loukota†, C. Ribera†, U. Jondelius‡, J. Bagun˜ a` *, and M. Riutort*§ *Departament de Gene`tica and †Departament de Biologia Animal, Universitat Barcelona, Av. Diagonal, 645 08028 Barcelona, Spain; and ‡Evolutionary Biology Centre, University Uppsala, Norbyva¨gen 18D, 752 36 Uppsala, Sweden Communicated by James W. Valentine, University of California, Berkeley, CA, June 28, 2002 (received for review April 15, 2002) Bilateria are currently subdivided into three superclades: Deuter- across all bilaterians. However different, this scheme also suited ostomia, Ecdysozoa, and Lophotrochozoa. Within this new taxo- alternative hypotheses such as the ‘‘set-aside cells’’ theory (13, nomic frame, acoelomate Platyhelminthes, for a long time held to 14) and the colonial ancestor theory (15). be basal bilaterians, are now considered spiralian lophotrochozo- This new status quo was soon questioned. A SSU-based study ans. However, recent 18S rDNA [small subunit (SSU)] analyses have using a large set of Platyhelminthes acoels and other metazoans shown Platyhelminthes to be polyphyletic with two of its orders, showed acoels to be the extant earliest branching bilaterians the Acoela and the Nemertodermatida, as the earliest extant (16), turning Platyhelminthes into a polyphyletic group. In bilaterians. To corroborate such position and avoid the criticisms of addition, Jenner (17) noted that phylogenies put forward to back saturation and long-branch effects thrown on the SSU molecule, the new metazoan molecular trees (10–12, 18) were incomplete we have searched for independent molecular data bearing good and heavily pruned. -

New and Known Nemertodermatida (Platyhelminthes-Acoelomorpha)
Belg. J. Zoo!. - Volume 128 (1998) - issue 1 - pages 55-92 - Brussels 1998 Received: 18 February 1998 NEW AND KNOWN NEMERTODERMATIDA (PLATYHELMINTHES-ACOELOMORPHA)... -A REVISION - WOLFGANG STERRER Bermuda Natural History Museum, Flatts FLBX, Bermuda e-mail [email protected] Abstract. Described in 1930-31 by Steinbiick who considered it the most primitive bilaterian, the turbellarian genus Nemertoderma is known for its rote in platyhelminth phylogeny as much as for its muddled taxonomy. On the basis of material collected in the Mediterranean, Atlantic and Pacifie Oceans sin ce 1964 this paper re-diagnoses the known 4 genera and 7 species (Nemertoderma bathycola Steinbiick, 1930-31 ; N. westbladi Steinbiick, 1938; N. psammicola Sterrer, 1970 (syn. N. rubra Faubel, 1976); Meara stichopi Westblad, 1949; Meara sp. (see SMITH et al., 1994); Nemertinoides elongatus Riser, 1987 ; and Flagellophora apelti Fau bel & Diirjes, 1978), describes one new genus with 2 new species (Ascoparia neglecta n. g., n. sp. and A. secunda n. sp.), and pro vides observations from living material on morphological variability, body size vs . reproductive state, statocyst structure and statolith variability, and sperm morphology and dimorphism. The paper concludes with diagnoses for the known taxa of Nemertodermatida, including the new family Ascopariidae. Key words: Platyhelminthes, free-living, marine; systematics, new species. INTRODUCTION ln 1930-31 Otto STEfNBOCK described Nemertoderma bathycola from a single tiny worm whicb he and Erich Reisinger bad dredged from a muddy bottom, at 300-400 rn depth, off Greenland. Nemertoderma caused a small sensation, not only because Steinbock, a meticulous observer, was also an assertive character (who liked to express himself double-spaced, with exclamation marks added) but because Nemertoderma was indeed unusual. -

Carbonic Anhydrases: an Ancient Tool in Calcareous Sponge Biomineralization
fgene-12-624533 March 30, 2021 Time: 13:30 # 1 BRIEF RESEARCH REPORT published: 07 April 2021 doi: 10.3389/fgene.2021.624533 Carbonic Anhydrases: An Ancient Tool in Calcareous Sponge Biomineralization Oliver Voigt1*, Benedetta Fradusco1, Carolin Gut1, Charalampos Kevrekidis1, Sergio Vargas1 and Gert Wörheide1,2,3 1 Department of Earth and Environmental Sciences, Palaeontology and Geobiology, Ludwig-Maximilians-Universität München, Munich, Germany, 2 GeoBio-Center, Ludwig-Maximilians-Universität München, Munich, Germany, 3 SNSB-Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany Enzymes of the a-carbonic anhydrase gene family (CAs) are essential for the deposition of calcium carbonate biominerals. In calcareous sponges (phylum Porifera, class Calcarea), specific CAs are involved in the formation of calcite spicules, a unique trait and synapomorphy of this class. However, detailed studies on the CA repertoire of calcareous sponges exist for only two species of one of the two Calcarea subclasses, the Calcaronea. The CA repertoire of the second subclass, the Calcinea, has not been investigated so far, leaving a considerable gap in our knowledge about this Edited by: Melanie Debiais-Thibaud, gene family in Calcarea. Here, using transcriptomic analysis, phylogenetics, and in situ Université de Montpellier, France hybridization, we study the CA repertoire of four additional species of calcareous Reviewed by: sponges, including three from the previously unsampled subclass Calcinea. Our data Helena Cetkovi´ c,´ Rudjer Boskovic Institute, Croatia indicate that the last common ancestor of Calcarea had four ancestral CAs with defined Ana Riesgo, subcellular localizations and functions (mitochondrial/cytosolic, membrane-bound, and Natural History Museum, secreted non-catalytic). The evolution of membrane-bound and secreted CAs involved United Kingdom gene duplications and losses, whereas mitochondrial/cytosolic and non-catalytic CAs *Correspondence: Oliver Voigt are evidently orthologous genes. -
S I Section 4
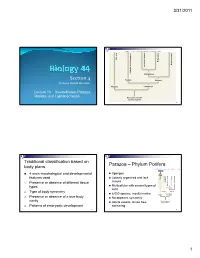
3/31/2011 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Porifera Ecdysozoa Deuterostomia Lophotrochozoa Cnidaria and Ctenophora Cnidaria and Protostomia SSiection 4 Radiata Bilateria Professor Donald McFarlane Parazoa Eumetazoa Lecture 13 Invertebrates: Parazoa, Radiata, and Lophotrochozoa Ancestral colonial choanoflagellate 2 Traditional classification based on Parazoa – Phylum Porifera body plans Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Parazoa 4 main morphological and developmental Sponges features used Loosely organized and lack Porifera Ecdysozoa Cnidaria and Ctenophora tissues euterostomia photrochozoa D 1. o Presence or absence of different tissue L Multicellular with several types of types Protostomia cells 2. Type of body symmetry Radiata Bilateria 8,000 species, mostly marine Parazoa Eumetazoa 3. Presence or absence of a true body No apparent symmetry cavity Ancestral colonial Adults sessile, larvae free- choanoflagellate 4. Patterns of embryonic development swimming 3 4 1 3/31/2011 Water drawn through pores (ostia) into spongocoel Flows out through osculum Reproduce Choanocytes line spongocoel Sexually Most hermapppgggphrodites producing eggs and sperm Trap and eat small particles and plankton Gametes are derived from amoebocytes or Mesohyl between choanocytes and choanocytes epithelial cells Asexually Amoebocytes absorb food from choanocytes, Small fragment or bud may detach and form a new digest it, and carry -

Molecular Homology & the Ancient Genetic Toolkit: How Evolutionary
Regis University ePublications at Regis University All Regis University Theses Spring 2019 Molecular Homology & the Ancient Genetic Toolkit: How Evolutionary Development Could Shape Your Next Doctor's Appointment Elizabeth G. Plender Follow this and additional works at: https://epublications.regis.edu/theses Part of the Cell Biology Commons, Developmental Biology Commons, Evolution Commons, Molecular Biology Commons, and the Molecular Genetics Commons Recommended Citation Plender, Elizabeth G., "Molecular Homology & the Ancient Genetic Toolkit: How Evolutionary Development Could Shape Your Next Doctor's Appointment" (2019). All Regis University Theses. 905. https://epublications.regis.edu/theses/905 This Thesis - Open Access is brought to you for free and open access by ePublications at Regis University. It has been accepted for inclusion in All Regis University Theses by an authorized administrator of ePublications at Regis University. For more information, please contact [email protected]. MOLECULAR HOMOLOGY AND THE ANCIENT GENETIC TOOLKIT: HOW EVOLUTIONARY DEVELOPMENT COULD SHAPE YOUR NEXT DOCTOR’S APPOINTMENT A thesis submitted to Regis College The Honors Program In partial fulfillment of the requirements For Graduation with Honors By Elizabeth G. Plender May 2019 Thesis written by Elizabeth Plender Approved by Thesis Advisor Thesis Reader Accepted by Director, University Honors Program ii iii TABLE OF CONTENTS List of Figures ............................................................................................................................................... -

Basal Metazoans - Dirk Erpenbeck, Simion Paul, Michael Manuel, Paulyn Cartwright, Oliver Voigt and Gert Worheide
EVOLUTION OF PHYLOGENETIC TREE OF LIFE - Basal Metazoans - Dirk Erpenbeck, Simion Paul, Michael Manuel, Paulyn Cartwright, Oliver Voigt and Gert Worheide BASAL METAZOANS Dirk Erpenbeck Ludwig-Maximilians Universität München, Germany Simion Paul and Michaël Manuel Université Pierre et Marie Curie in Paris, France. Paulyn Cartwright University of Kansas USA. Oliver Voigt and Gert Wörheide Ludwig-Maximilians Universität München, Germany Keywords: Metazoa, Porifera, sponges, Placozoa, Cnidaria, anthozoans, jellyfishes, Ctenophora, comb jellies Contents 1. Introduction on ―Basal Metazoans‖ 2. Phylogenetic relationships among non-bilaterian Metazoa 3. Porifera (Sponges) 4. Placozoa 5. Ctenophora (Comb-jellies) 6. Cnidaria 7. Cultural impact and relevance to human welfare Glossary Bibliography Biographical Sketch Summary Basal metazoans comprise the four non-bilaterian animal phyla Porifera (sponges), Cnidaria (anthozoans and jellyfishes), Placozoa (Trichoplax) and Ctenophora (comb jellies). The phylogenetic position of these taxa in the animal tree is pivotal for our understanding of the last common metazoan ancestor and the character evolution all Metazoa,UNESCO-EOLSS but is much debated. Morphological, evolutionary, internal and external phylogenetic aspects of the four phyla are highlighted and discussed. SAMPLE CHAPTERS 1. Introduction on “Basal Metazoans” In many textbooks the term ―lower metazoans‖ still refers to an undefined assemblage of invertebrate phyla, whose phylogenetic relationships were rather undefined. This assemblage may contain both bilaterian and non-bilaterian taxa. Currently, ―Basal Metazoa‖ refers to non-bilaterian animals only, four phyla that lack obvious bilateral symmetry, Porifera, Placozoa, Cnidaria and Ctenophora. ©Encyclopedia of Life Support Systems (EOLSS) EVOLUTION OF PHYLOGENETIC TREE OF LIFE - Basal Metazoans - Dirk Erpenbeck, Simion Paul, Michael Manuel, Paulyn Cartwright, Oliver Voigt and Gert Worheide These four phyla have classically been known as ―diploblastic‖ Metazoa. -

Xenacoelomorpha Atworms Are Basal Deuterostome
Xenacoelomorpha atworms are basal Deuterostome Yi Wang ( [email protected] ) Research article Keywords: Xenacoelomorpha atworm, Darwin’s “tree of life” Posted Date: August 28th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-64037/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/11 Abstract Background: Whether position Xenacoelomorpha as an early branch of Bilateria (Protostomes + Deuterostomes) has been intensely debated during last several decades. Considering Darwin’s “tree of life”, with the “Phylogenetic Species Concept”, we choose mitochondrial genome as the subject to predict phylogenetic position of Xenacoelomorpha, by genes genealogy. Results: Herein, we sequence Heterochaerus australis’s mitochondrial genome and infer intrinsic relationships of Metazoan with Xenacoelomorpha. The optimal tree under the popular maximum likelihood (ML) and Bayesian phylogenetic reconstructions are consensus with each other being strongly supported. The relationship between Chordates, Ambulacrarians and Xenoturbella/Acoelomorph is resolved. To avoid previous query about alignment process, the datasets are alignmented and trimmed automatically. Reducing taxon or cutting outgroups can not affect the relationship between Xenacoelomorpha and other Metazoan. Meanwhile, analysis using CAT model and Dayhoff groups also supporting the prediction made by mtZOA, relaxing the restriction of alignment criteria ( MAFFT, strategy G–ins–1, BLOSUM 62, 45, 30 ) introducing potential misleading -

First Record of Nemertodermatida from Belgian Marine Waters
Belg. J. Zool., 141 (1) : 62-64 January 2011 First record of Nemertodermatida from Belgian marine waters Mieke Boone1*, Wouter Houthoofd1, Wim Bert1 & Tom Artois2 1 Ghent University, Department Biology, Nematology Unit, K.L. Ledeganckstraat 35, B-9000 Gent, Belgium 2 Hasselt University, Centre for Environmental Sciences, Research Group Zoology: Biodiversity & Toxicology, Agoralaan Gebouw D, B-3590 Diepenbeek, Belgium * Corresponding author: [email protected] delimitation is often problematic and also cryptic diver- KEY WORDS: Acoelomorpha, Nemertodermatida, new sity cannot be excluded, which makes nemertodermatids records, Belgium also interesting from a (molecular) taxonomical point of view. Regrettably, Nemertodermatida are known from a few distinct sampling spots only: the Swedish west coast, the east coast of North America, the Adriatic and the Nemertodermatida is a small taxon of marine worms, Mediterranean, which suggests that representatives of the comprising only eight described species. They are charac- taxon are difficult to collect. terised by a nemertine-like epidermis (hence the name) The Belgian Continental Shelf, situated in the southern and a statocyst containing (mostly) two statoliths (1). The bight of the North Sea and characterised by a mainly phylogenetic relationships of the taxon have been (and sandy substrate, has not previously been sampled for tur- still are) the subject of debate. Initially, they were placed bellarians specifically, in contrast to the sandy beaches of within the acoel Platyhelminthes (2), but Karling (1, 3) the Belgian coast, the turbellarian fauna of which is very considered them to be substantially different from the well known (15 ; 16). Recently, during a two-year period acoels in morphology of the gut and the statocyst, and (2007 – 2009), researchers from the UHasselt and UGent placed the taxon Nemertodermatida outside the Acoela sampled the Belgian continental shelf, in order to investi- (3).