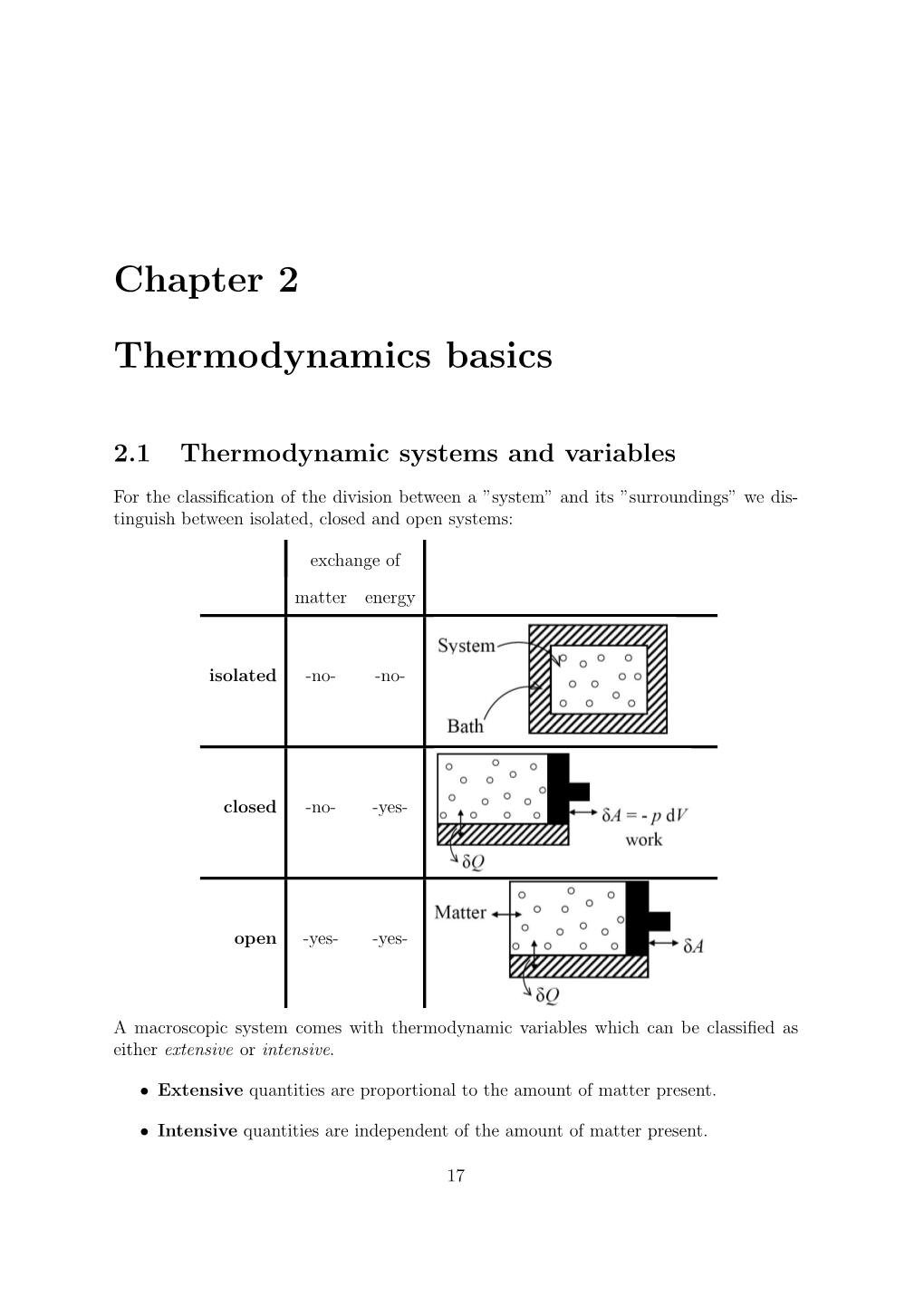
Chapter 2 Thermodynamics Basics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -

Thermodynamic State Variables Gunt
Fundamentals of thermodynamics 1 Thermodynamic state variables gunt Basic knowledge Thermodynamic state variables Thermodynamic systems and principles Change of state of gases In physics, an idealised model of a real gas was introduced to Equation of state for ideal gases: State variables are the measurable properties of a system. To make it easier to explain the behaviour of gases. This model is a p × V = m × Rs × T describe the state of a system at least two independent state system boundaries highly simplifi ed representation of the real states and is known · m: mass variables must be given. surroundings as an “ideal gas”. Many thermodynamic processes in gases in · Rs: spec. gas constant of the corresponding gas particular can be explained and described mathematically with State variables are e.g.: the help of this model. system • pressure (p) state process • temperature (T) • volume (V) Changes of state of an ideal gas • amount of substance (n) Change of state isochoric isobaric isothermal isentropic Condition V = constant p = constant T = constant S = constant The state functions can be derived from the state variables: Result dV = 0 dp = 0 dT = 0 dS = 0 • internal energy (U): the thermal energy of a static, closed Law p/T = constant V/T = constant p×V = constant p×Vκ = constant system. When external energy is added, processes result κ =isentropic in a change of the internal energy. exponent ∆U = Q+W · Q: thermal energy added to the system · W: mechanical work done on the system that results in an addition of heat An increase in the internal energy of the system using a pressure cooker as an example. -

Basic Thermodynamics-17ME33.Pdf
Module -1 Fundamental Concepts & Definitions & Work and Heat MODULE 1 Fundamental Concepts & Definitions Thermodynamics definition and scope, Microscopic and Macroscopic approaches. Some practical applications of engineering thermodynamic Systems, Characteristics of system boundary and control surface, examples. Thermodynamic properties; definition and units, intensive and extensive properties. Thermodynamic state, state point, state diagram, path and process, quasi-static process, cyclic and non-cyclic processes. Thermodynamic equilibrium; definition, mechanical equilibrium; diathermic wall, thermal equilibrium, chemical equilibrium, Zeroth law of thermodynamics, Temperature; concepts, scales, fixed points and measurements. Work and Heat Mechanics, definition of work and its limitations. Thermodynamic definition of work; examples, sign convention. Displacement work; as a part of a system boundary, as a whole of a system boundary, expressions for displacement work in various processes through p-v diagrams. Shaft work; Electrical work. Other types of work. Heat; definition, units and sign convention. 10 Hours 1st Hour Brain storming session on subject topics Thermodynamics definition and scope, Microscopic and Macroscopic approaches. Some practical applications of engineering thermodynamic Systems 2nd Hour Characteristics of system boundary and control surface, examples. Thermodynamic properties; definition and units, intensive and extensive properties. 3rd Hour Thermodynamic state, state point, state diagram, path and process, quasi-static -

Thermal Equilibrium State of the World Oceans
Thermal equilibrium state of the ocean Rui Xin Huang Department of Physical Oceanography Woods Hole Oceanographic Institution Woods Hole, MA 02543, USA April 24, 2010 Abstract The ocean is in a non-equilibrium state under the external forcing, such as the mechanical energy from wind stress and tidal dissipation, plus the huge amount of thermal energy from the air-sea interface and the freshwater flux associated with evaporation and precipitation. In the study of energetics of the oceanic circulation it is desirable to examine how much energy in the ocean is available. In order to calculate the so-called available energy, a reference state should be defined. One of such reference state is the thermal equilibrium state defined in this study. 1. Introduction Chemical potential is a part of the internal energy. Thermodynamics of a multiple component system can be established from the definition of specific entropy η . Two other crucial variables of a system, including temperature and specific chemical potential, can be defined as follows 1 ⎛⎞∂η ⎛⎞∂η = ⎜⎟, μi =−Tin⎜⎟, = 1,2,..., , (1) Te m ⎝⎠∂ vm, i ⎝⎠∂ i ev, where e is the specific internal energy, v is the specific volume, mi and μi are the mass fraction and chemical potential for the i-th component. For a multiple component system, the change in total chemical potential is the sum of each component, dc , where c is the mass fraction of each component. The ∑i μii i mass fractions satisfy the constraint c 1 . Thus, the mass fraction of water in sea ∑i i = water satisfies dc=− c , and the total chemical potential for sea water is wi∑iw≠ N −1 ∑()μμiwi− dc . -

Laws of Thermodynamics
Advanced Instructional School on Mechanics, 5 - 24, Dec 2011 Special lectures Laws of Thermodynamics K. P. N. Murthy School of Physics, University of Hyderabad Dec 15, 2011 K P N Murthy (UoH) Thermodynamics Dec 15, 2011 1 / 126 acknowledgement and warning acknowledgement: Thanks to Prof T Amaranath and V D Sharma for the invitation warning: I am going to talk about the essential contents of the laws of thermodynamics, tracing their origin, and their history The Zeroth law, the First law the Second law .....and may be the Third law, if time permits I leave it to your imagination to connect my talks to the theme of the School which is MECHANICS. K P N Murthy (UoH) Thermodynamics Dec 15, 2011 2 / 126 Each law provides an experimental basis for a thermodynamic property Zeroth law= ) Temperature, T First law= ) Internal Energy, U Second law= ) Entropy, S The earliest was the Second law discovered in the year 1824 by Sadi Carnot (1796-1832) Sadi Carnot Helmholtz Rumford Mayer Joule then came the First law - a few decades later, when Helmholtz consolidated and abstracted the experimental findings of Rumford, Mayer and Joule into a law. the Zeroth law arrived only in the twentieth century, and I think Max Planck was responsible for it K P N Murthy (UoH) Thermodynamics Dec 15, 2011 3 / 126 VOCALUBARY System: The small part of the universe under consideration: e.g. a piece of iron a glass of water an engine The rest of the universe (in which we stand and make observations and measurements on the system) is called the surroundings a glass of water is the system and the room in which the glass is placed is called the surrounding. -

Thermodynamic State, Specific Heat, and Enthalpy Function 01 Saturated UO Z Vapor Between 3000 Kand 5000 K
Februar 1977 KFK 2390 Institut für Neutronenphysik und Reaktortechnik Projekt Schneller Brüter Thermodynamic State, Specific Heat, and Enthalpy Function 01 Saturated UO z Vapor between 3000 Kand 5000 K H. U. Karow Als Manuskript vervielfältigt Für diesen Bericht behalten wir uns alle Rechte vor GESELLSCHAFT FÜR KERNFORSCHUNG M. B. H. KARLSRUHE KERNFORSCHUNGS ZENTRUM KARLSRUHE KFK 2390 Institut für Neutronenphysik und Reaktortechnik Projekt Schneller Brüter Thermodynamic State, Specific Heat, and Enthalpy Function of Saturated U0 Vapor between 3000 K 2 and 5000 K H. U. Karow Gesellschaft für Kernforschung mbH., Karlsruhe A summarized version of s will be at '7th Symposium on Thermophysical Properties', held the NBS at Gaithe , 1977 Thermodynamic State, Specific Heat, and Enthalpy Function of Saturated U0 2 Vapor between 3000 K and 5000 K Abstract Reactor safety analysis requires knowledge of the thermophysical properties of molten oxide fuel and of the thermal equation-of state of oxide fuel in thermodynamic liquid-vapor equilibrium far above 3000 K. In this context, the thermodynamic state of satu rated U0 2 fuel vapor, its internal energy U(T), specific heats Cv(T) and C (T), and enthalpy functions HO(T) and HO(T)-Ho have p 0 been determined by means of statistical mechanics in the tempera- ture range 3000 K .•• 5000 K. The discussion of the thermodynamic state includes the evaluation of the plasma state and its contri bution to the caloric variables-of-state of saturated oxide fuel vapor. Because of the extremely high ion and electron density due to thermal ionization, the ionized component of the fuel vapor does no more represent a perfect kinetic plasma - different from the nonionized neutral vapor component with perfect gas kinetic behavior up to about 5000 K. -

Chapter 5 the Laws of Thermodynamics: Entropy, Free
Chapter 5 The laws of thermodynamics: entropy, free energy, information and complexity M.W. Collins1, J.A. Stasiek2 & J. Mikielewicz3 1School of Engineering and Design, Brunel University, Uxbridge, Middlesex, UK. 2Faculty of Mechanical Engineering, Gdansk University of Technology, Narutowicza, Gdansk, Poland. 3The Szewalski Institute of Fluid–Flow Machinery, Polish Academy of Sciences, Fiszera, Gdansk, Poland. Abstract The laws of thermodynamics have a universality of relevance; they encompass widely diverse fields of study that include biology. Moreover the concept of information-based entropy connects energy with complexity. The latter is of considerable current interest in science in general. In the companion chapter in Volume 1 of this series the laws of thermodynamics are introduced, and applied to parallel considerations of energy in engineering and biology. Here the second law and entropy are addressed more fully, focusing on the above issues. The thermodynamic property free energy/exergy is fully explained in the context of examples in science, engineering and biology. Free energy, expressing the amount of energy which is usefully available to an organism, is seen to be a key concept in biology. It appears throughout the chapter. A careful study is also made of the information-oriented ‘Shannon entropy’ concept. It is seen that Shannon information may be more correctly interpreted as ‘complexity’rather than ‘entropy’. We find that Darwinian evolution is now being viewed as part of a general thermodynamics-based cosmic process. The history of the universe since the Big Bang, the evolution of the biosphere in general and of biological species in particular are all subject to the operation of the second law of thermodynamics. -

Chapter 20 -- Thermodynamics
ChapterChapter 2020 -- ThermodynamicsThermodynamics AA PowerPointPowerPoint PresentationPresentation byby PaulPaul E.E. TippensTippens,, ProfessorProfessor ofof PhysicsPhysics SouthernSouthern PolytechnicPolytechnic StateState UniversityUniversity © 2007 THERMODYNAMICSTHERMODYNAMICS ThermodynamicsThermodynamics isis thethe studystudy ofof energyenergy relationshipsrelationships thatthat involveinvolve heat,heat, mechanicalmechanical work,work, andand otherother aspectsaspects ofof energyenergy andand heatheat transfer.transfer. Central Heating Objectives:Objectives: AfterAfter finishingfinishing thisthis unit,unit, youyou shouldshould bebe ableable to:to: •• StateState andand applyapply thethe first andand second laws ofof thermodynamics. •• DemonstrateDemonstrate youryour understandingunderstanding ofof adiabatic, isochoric, isothermal, and isobaric processes.processes. •• WriteWrite andand applyapply aa relationshiprelationship forfor determiningdetermining thethe ideal efficiency ofof aa heatheat engine.engine. •• WriteWrite andand applyapply aa relationshiprelationship forfor determiningdetermining coefficient of performance forfor aa refrigeratior.refrigeratior. AA THERMODYNAMICTHERMODYNAMIC SYSTEMSYSTEM •• AA systemsystem isis aa closedclosed environmentenvironment inin whichwhich heatheat transfertransfer cancan taketake place.place. (For(For example,example, thethe gas,gas, walls,walls, andand cylindercylinder ofof anan automobileautomobile engine.)engine.) WorkWork donedone onon gasgas oror workwork donedone byby gasgas INTERNALINTERNAL -

A Reference Equation of State for the Thermodynamic Properties of Nitrogen for Temperatures from 63.151 to 1000 K and Pressures to 2200 Mpa Roland Span, Eric W
A Reference Equation of State for the Thermodynamic Properties of Nitrogen for Temperatures from 63.151 to 1000 K and Pressures to 2200 MPa Roland Span, Eric W. Lemmon, Richard T Jacobsen, Wolfgang Wagner, and Akimichi Yokozeki Citation: Journal of Physical and Chemical Reference Data 29, 1361 (2000); doi: 10.1063/1.1349047 View online: http://dx.doi.org/10.1063/1.1349047 View Table of Contents: http://scitation.aip.org/content/aip/journal/jpcrd/29/6?ver=pdfcov Published by the AIP Publishing Articles you may be interested in A Reference Equation of State for the Thermodynamic Properties of Ethane for Temperatures from the Melting Line to 675 K and Pressures up to 900 MPa J. Phys. Chem. Ref. Data 35, 205 (2006); 10.1063/1.1859286 A New Functional Form and New Fitting Techniques for Equations of State with Application to Pentafluoroethane (HFC-125) J. Phys. Chem. Ref. Data 34, 69 (2005); 10.1063/1.1797813 The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use J. Phys. Chem. Ref. Data 31, 387 (2002); 10.1063/1.1461829 New Equation of State for Ethylene Covering the Fluid Region for Temperatures From the Melting Line to 450 K at Pressures up to 300 MPa J. Phys. Chem. Ref. Data 29, 1053 (2000); 10.1063/1.1329318 Thermodynamic Properties of Air and Mixtures of Nitrogen, Argon, and Oxygen From 60 to 2000 K at Pressures to 2000 MPa J. Phys. Chem. Ref. Data 29, 331 (2000); 10.1063/1.1285884 This article is copyrighted as indicated in the article. -

A Specific Heat Ratio Model and Compression Ratio Estimation
Linköping Studies in Science and Technology Thesis No. 1104 A Specific Heat Ratio Model and Compression Ratio Estimation Marcus Klein Division of Vehicular Systems Department of Electrical Engineering Linköping University, SE–581 83 Linköping, Sweden http://www.vehicular.isy.liu.se/ Email: [email protected] Linköping 2004 A Specific Heat Ratio Model and Compression Ratio Estimation °c 2004 Marcus Klein Department of Electrical Engineering, Linköping University, SE–581 83 Linköping, Sweden. ISBN 91-7373-992-8 ISSN 0280-7971 LiU-TEK-LIC-2004:33 Printed by UniTryck, Linköping, Sweden 2004 To Sofia and Ebba Abstract Cylinder pressure modeling and heat release analysis are today important and standard tools for engineers and researchers, when developing and tuning new engines. An accurate specific heat ratio model is important for an accurate heat release analysis, since the specific heat ratio couples the systems energy to other thermodynamic quantities. The objective of the first part is therefore to investigate models of the specific heat ratio for the single-zone heat release model, and find a model accurate enough to introduce a cylinder pressure modeling error less than or in the order of the cylinder pressure measurement noise, while keeping the computational complexity at a minimum. As reference, a specific heat ratio is calculated for burned and unburned gases, assuming that the unburned mixture is frozen and that the burned is at chemical equilibrium. Use of the reference model in heat release analysis is too time consuming and therefore a set of simpler models, both existing and newly developed, are compared to the reference model. -

Thermodynamics
CHAPTER TWELVE THERMODYNAMICS 12.1 INTRODUCTION In previous chapter we have studied thermal properties of matter. In this chapter we shall study laws that govern thermal energy. We shall study the processes where work is 12.1 Introduction converted into heat and vice versa. In winter, when we rub 12.2 Thermal equilibrium our palms together, we feel warmer; here work done in rubbing 12.3 Zeroth law of produces the ‘heat’. Conversely, in a steam engine, the ‘heat’ Thermodynamics of the steam is used to do useful work in moving the pistons, 12.4 Heat, internal energy and which in turn rotate the wheels of the train. work In physics, we need to define the notions of heat, 12.5 First law of temperature, work, etc. more carefully. Historically, it took a thermodynamics long time to arrive at the proper concept of ‘heat’. Before the 12.6 Specific heat capacity modern picture, heat was regarded as a fine invisible fluid 12.7 Thermodynamic state filling in the pores of a substance. On contact between a hot variables and equation of body and a cold body, the fluid (called caloric) flowed from state the colder to the hotter body! This is similar to what happens 12.8 Thermodynamic processes when a horizontal pipe connects two tanks containing water 12.9 Heat engines up to different heights. The flow continues until the levels of 12.10 Refrigerators and heat water in the two tanks are the same. Likewise, in the ‘caloric’ pumps picture of heat, heat flows until the ‘caloric levels’ (i.e., the 12.11 Second law of temperatures) equalise. -

A Dynamical Systems Theory of Thermodynamics
© Copyright, Princeton University Press. No part of this book may be distributed, posted, or reproduced in any form by digital or mechanical means without prior written permission of the publisher. Chapter One Introduction 1.1 An Overview of Classical Thermodynamics Energy is a concept that underlies our understanding of all physical phenomena and is a measure of the ability of a dynamical system to produce changes (motion) in its own system state as well as changes in the system states of its surroundings. Thermodynamics is a physical branch of science that deals with laws governing energy flow from one body to another and energy transformations from one form to another. These energy flow laws are captured by the fundamental principles known as the first and second laws of thermodynamics. The first law of thermodynamics gives a precise formulation of the equivalence between heat (i.e., the transferring of energy via temperature gradients) and work (i.e., the transferring of energy into coherent motion) and states that, among all system transformations, the net system energy is conserved. Hence, energy cannot be created out of nothing and cannot be destroyed; it can merely be transferred from one form to another. The law of conservation of energy is not a mathematical truth, but rather the consequence of an immeasurable culmination of observations over the chronicle of our civilization and is a fundamental axiom of the science of heat. The first law does not tell us whether any particular process can actually occur, that is, it does not restrict the ability to convert work into heat or heat into work, except that energy must be conserved in the process.