Thermodynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -

Equilibrium Thermodynamics
Equilibrium Thermodynamics Instructor: - Clare Yu (e-mail [email protected], phone: 824-6216) - office hours: Wed from 2:30 pm to 3:30 pm in Rowland Hall 210E Textbooks: - primary: Herbert Callen “Thermodynamics and an Introduction to Thermostatistics” - secondary: Frederick Reif “Statistical and Thermal Physics” - secondary: Kittel and Kroemer “Thermal Physics” - secondary: Enrico Fermi “Thermodynamics” Grading: - weekly homework: 25% - discussion problems: 10% - midterm exam: 30% - final exam: 35% Equilibrium Thermodynamics Material Covered: Equilibrium thermodynamics, phase transitions, critical phenomena (~ 10 first chapters of Callen’s textbook) Homework: - Homework assignments posted on course website Exams: - One midterm, 80 minutes, Tuesday, May 8 - Final, 2 hours, Tuesday, June 12, 10:30 am - 12:20 pm - All exams are in this room 210M RH Course website is at http://eiffel.ps.uci.edu/cyu/p115B/class.html The Subject of Thermodynamics Thermodynamics describes average properties of macroscopic matter in equilibrium. - Macroscopic matter: large objects that consist of many atoms and molecules. - Average properties: properties (such as volume, pressure, temperature etc) that do not depend on the detailed positions and velocities of atoms and molecules of macroscopic matter. Such quantities are called thermodynamic coordinates, variables or parameters. - Equilibrium: state of a macroscopic system in which all average properties do not change with time. (System is not driven by external driving force.) Why Study Thermodynamics ? - Thermodynamics predicts that the average macroscopic properties of a system in equilibrium are not independent from each other. Therefore, if we measure a subset of these properties, we can calculate the rest of them using thermodynamic relations. - Thermodynamics not only gives the exact description of the state of equilibrium but also provides an approximate description (to a very high degree of precision!) of relatively slow processes. -
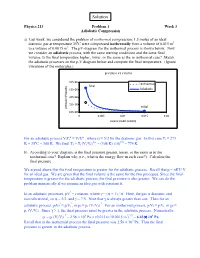
3-1 Adiabatic Compression
Solution Physics 213 Problem 1 Week 3 Adiabatic Compression a) Last week, we considered the problem of isothermal compression: 1.5 moles of an ideal diatomic gas at temperature 35oC were compressed isothermally from a volume of 0.015 m3 to a volume of 0.0015 m3. The pV-diagram for the isothermal process is shown below. Now we consider an adiabatic process, with the same starting conditions and the same final volume. Is the final temperature higher, lower, or the same as the in isothermal case? Sketch the adiabatic processes on the p-V diagram below and compute the final temperature. (Ignore vibrations of the molecules.) α α For an adiabatic process ViTi = VfTf , where α = 5/2 for the diatomic gas. In this case Ti = 273 o 1/α 2/5 K + 35 C = 308 K. We find Tf = Ti (Vi/Vf) = (308 K) (10) = 774 K. b) According to your diagram, is the final pressure greater, lesser, or the same as in the isothermal case? Explain why (i.e., what is the energy flow in each case?). Calculate the final pressure. We argued above that the final temperature is greater for the adiabatic process. Recall that p = nRT/ V for an ideal gas. We are given that the final volume is the same for the two processes. Since the final temperature is greater for the adiabatic process, the final pressure is also greater. We can do the problem numerically if we assume an idea gas with constant α. γ In an adiabatic processes, pV = constant, where γ = (α + 1) / α. -

Thermodynamics of Solar Energy Conversion in to Work
Sri Lanka Journal of Physics, Vol. 9 (2008) 47-60 Institute of Physics - Sri Lanka Research Article Thermodynamic investigation of solar energy conversion into work W.T.T. Dayanga and K.A.I.L.W. Gamalath Department of Physics, University of Colombo, Colombo 03, Sri Lanka Abstract Using a simple thermodynamic upper bound efficiency model for the conversion of solar energy into work, the best material for a converter was obtained. Modifying the existing detailed terrestrial application model of direct solar radiation to include an atmospheric transmission coefficient with cloud factors and a maximum concentration ratio, the best shape for a solar concentrator was derived. Using a Carnot engine in detailed space application model, the best shape for the mirror of a concentrator was obtained. A new conversion model was introduced for a solar chimney power plant to obtain the efficiency of the power plant and power output. 1. INTRODUCTION A system that collects and converts solar energy in to mechanical or electrical power is important from various aspects. There are two major types of solar power systems at present, one using photovoltaic cells for direct conversion of solar radiation energy in to electrical energy in combination with electrochemical storage and the other based on thermodynamic cycles. The efficiency of a solar thermal power plant is significantly higher [1] compared to the maximum efficiency of twenty percent of a solar cell. Although the initial cost of a solar thermal power plant is very high, the running cost is lower compared to the other power plants. Therefore most countries tend to build solar thermal power plants. -

Guggenheim Aeronautical Laboratory~://; California
R.ES~.LttCH REPCRT GUGGENHEIM AERONAUTICAL LABORATORY ~://; CALIFORNIA INSTITUTE OF TECHNOLOGY HYPERSC»JIC RESEARCH PROJECT Memorandum No. 47 December 15, 1958 AN EXPERIMENTAL INVESTIGATION OF THE EFFECT OF EJECTING A COOLANT GAS AT THE NOSE OF A BLUNT BODY by C. Hugh E. Warren ARMY ORDNANCE CONTRACT NO. DA-04-495-0rd-19 GUGGENHEIM AERONAUTICAL LABORATORY CALIFORNIA INSTITUTE OF TECHNOLOGY Pasadena1 California HYPERSONIC RESEARCH PROJECT Memorandum No. 4 7 December 151 1958 AN EXPERIMENTAL INVESTIGATION OF THE EFFECT OF EJECTING A COOLANT GAS AT THE NOSE OF A BLUNT BODY by C. Hugh E. Warren ~~C!a r~:Ml ia:nl bil'eCtOr Guggenheim Aeronautical Laboratory ARMY ORDNANCE CONTRACT NO. DA-04-495-0rd-19 Army Project No. 5B0306004 Ordnance Project No. TB3-0118 OOR Project No. 1600-PE ACKNOWLEDGMENTS The author wishes to express his appreciation to Professor Lester Lees for his assistance1 guidance and encouragement during this work1 to Mr. Howard McDonald and other members of the Aeronautics Machine Shop for fabricating and maintaining the models and other items of equipment1 to Mr. Paul Baloga and the staff of the Hypersonic Wind Tunnel for their assistance and advice during testing~ to Mrs. Betty Laue for much computational work1 and to Mrs. Gerry Van Gieson for typing the manuscript and piloting the report through after the author 1 s departure. Most of the work was done while the author was in receipt of a Harkness Fellowship of the Commonwealth Fund of New York1 to whom thanks are due for the opportunity to spend a year on such an instructive and interesting program in such congenial surroundings. -

Solar Thermal Energy
22 Solar Thermal Energy Solar thermal energy is an application of solar energy that is very different from photovol- taics. In contrast to photovoltaics, where we used electrodynamics and solid state physics for explaining the underlying principles, solar thermal energy is mainly based on the laws of thermodynamics. In this chapter we give a brief introduction to that field. After intro- ducing some basics in Section 22.1, we will discuss Solar Thermal Heating in Section 22.2 and Concentrated Solar (electric) Power (CSP) in Section 22.3. 22.1 Solar thermal basics We start this section with the definition of heat, which sometimes also is called thermal energy . The molecules of a body with a temperature different from 0 K exhibit a disordered movement. The kinetic energy of this movement is called heat. The average of this kinetic energy is related linearly to the temperature of the body. 1 Usually, we denote heat with the symbol Q. As it is a form of energy, its unit is Joule (J). If two bodies with different temperatures are brought together, heat will flow from the hotter to the cooler body and as a result the cooler body will be heated. Dependent on its physical properties and temperature, this heat can be absorbed in the cooler body in two forms, sensible heat and latent heat. Sensible heat is that form of heat that results in changes in temperature. It is given as − Q = mC p(T2 T1), (22.1) where Q is the amount of heat that is absorbed by the body, m is its mass, Cp is its heat − capacity and (T2 T1) is the temperature difference. -

Thermodynamic State Variables Gunt
Fundamentals of thermodynamics 1 Thermodynamic state variables gunt Basic knowledge Thermodynamic state variables Thermodynamic systems and principles Change of state of gases In physics, an idealised model of a real gas was introduced to Equation of state for ideal gases: State variables are the measurable properties of a system. To make it easier to explain the behaviour of gases. This model is a p × V = m × Rs × T describe the state of a system at least two independent state system boundaries highly simplifi ed representation of the real states and is known · m: mass variables must be given. surroundings as an “ideal gas”. Many thermodynamic processes in gases in · Rs: spec. gas constant of the corresponding gas particular can be explained and described mathematically with State variables are e.g.: the help of this model. system • pressure (p) state process • temperature (T) • volume (V) Changes of state of an ideal gas • amount of substance (n) Change of state isochoric isobaric isothermal isentropic Condition V = constant p = constant T = constant S = constant The state functions can be derived from the state variables: Result dV = 0 dp = 0 dT = 0 dS = 0 • internal energy (U): the thermal energy of a static, closed Law p/T = constant V/T = constant p×V = constant p×Vκ = constant system. When external energy is added, processes result κ =isentropic in a change of the internal energy. exponent ∆U = Q+W · Q: thermal energy added to the system · W: mechanical work done on the system that results in an addition of heat An increase in the internal energy of the system using a pressure cooker as an example. -

Empirical Modelling of Einstein Absorption Refrigeration System
Journal of Advanced Research in Fluid Mechanics and Thermal Sciences 75, Issue 3 (2020) 54-62 Journal of Advanced Research in Fluid Mechanics and Thermal Sciences Journal homepage: www.akademiabaru.com/arfmts.html ISSN: 2289-7879 Empirical Modelling of Einstein Absorption Refrigeration Open Access System Keng Wai Chan1,*, Yi Leang Lim1 1 School of Mechanical Engineering, Engineering Campus, Universiti Sains Malaysia, 14300 Nibong Tebal, Penang, Malaysia ARTICLE INFO ABSTRACT Article history: A single pressure absorption refrigeration system was invented by Albert Einstein and Received 4 April 2020 Leo Szilard nearly ninety-year-old. The system is attractive as it has no mechanical Received in revised form 27 July 2020 moving parts and can be driven by heat alone. However, the related literature and Accepted 5 August 2020 work done on this refrigeration system is scarce. Previous researchers analysed the Available online 20 September 2020 refrigeration system theoretically, both the system pressure and component temperatures were fixed merely by assumption of ideal condition. These values somehow have never been verified by experimental result. In this paper, empirical models were proposed and developed to estimate the system pressure, the generator temperature and the partial pressure of butane in the evaporator. These values are important to predict the system operation and the evaporator temperature. The empirical models were verified by experimental results of five experimental settings where the power input to generator and bubble pump were varied. The error for the estimation of the system pressure, generator temperature and partial pressure of butane in evaporator are ranged 0.89-6.76%, 0.23-2.68% and 0.28-2.30%, respectively. -

Equilibrium, Energy Conservation, and Temperature Chapter Objectives
Equilibrium, Energy Conservation, and Temperature Chapter Objectives 1. Explain thermal equilibrium and how it relates to energy transport. 2. Understand temperature ranges important for biological systems and temperature sensing in mammals. 3. Understand temperature ranges important for the environment. 1. Thermal equilibrium and the Laws of thermodynamics Laws of Thermodynamics Energy of the system + = Constant Energy of surrounding Energy Conservation 1. Thermal equilibrium and the Laws of thermodynamics Energy conservation Rate of Rate of Rate of Rate of Energy In Energy Out Energy Generation Energy Storage 1. Thermal equilibrium and the Laws of thermodynamics Ex) Solid is put in the liquid. specific mass heat temperature Solid Liquid Energy = m( − ) 1. Thermal equilibrium and the Laws of thermodynamics Energy In = − Energy Out = 0 Energy Generation = 0 Energy Storage = ( +) − −( −) >> Use the numerical values as shown in last page’s picture and equation of energy conservation. Using energy conservation, we can find the final or equilibrium temperature. 2. Temperature in Living System Most biological activity is confined to a rather narrow temperature range of 0~60°C 2. Temperature in Living System Temperature Response to Human body How temperature affects the state of human body? - As shown in this picture, it is obvious that temperature needs to be controlled. 2. Temperature in Living System Temperature Sensation in Humans - The human being can perceive different gradations of cold and heat, as shown in this picture. 2. Temperature in Living System Thermal Comfort of Human and Animals - Body heat losses are affected by air temperature, humidity, velocity and other factors. - Especially, affected by humidity. 3. -

Novel Hot Air Engine and Its Mathematical Model – Experimental Measurements and Numerical Analysis
POLLACK PERIODICA An International Journal for Engineering and Information Sciences DOI: 10.1556/606.2019.14.1.5 Vol. 14, No. 1, pp. 47–58 (2019) www.akademiai.com NOVEL HOT AIR ENGINE AND ITS MATHEMATICAL MODEL – EXPERIMENTAL MEASUREMENTS AND NUMERICAL ANALYSIS 1 Gyula KRAMER, 2 Gabor SZEPESI *, 3 Zoltán SIMÉNFALVI 1,2,3 Department of Chemical Machinery, Institute of Energy and Chemical Machinery University of Miskolc, Miskolc-Egyetemváros 3515, Hungary e-mail: [email protected], [email protected], [email protected] Received 11 December 2017; accepted 25 June 2018 Abstract: In the relevant literature there are many types of heat engines. One of those is the group of the so called hot air engines. This paper introduces their world, also introduces the new kind of machine that was developed and built at Department of Chemical Machinery, Institute of Energy and Chemical Machinery, University of Miskolc. Emphasizing the novelty of construction and the working principle are explained. Also the mathematical model of this new engine was prepared and compared to the real model of engine. Keywords: Hot, Air, Engine, Mathematical model 1. Introduction There are three types of volumetric heat engines: the internal combustion engines; steam engines; and hot air engines. The first one is well known, because it is on zenith nowadays. The steam machines are also well known, because their time has just passed, even the elder ones could see those in use. But the hot air engines are forgotten. Our aim is to consider that one. The history of hot air engines is 200 years old. -

Application of Laser Doppler Velocimetry
APPLICATION OF LASER DOPPLER VELOCIMETRY (LDV) TO STUDY THE STRUCTURE OF GRAVITY CURRENTS UNDER FIRE CONDITIONS B Moghtaderi† Process Safety and Environment Protection Group Discipline of Chemical Engineering, School of Engineering Faculty of Engineering & Built Environment, The University of Newcastle Callaghan, NSW 2308, Australia ABSTRACT Gravity currents are of considerable safety importance primarily because of their role in the spread and transport of smoke and hot gases in building fires. Despite recent progress in the field, relatively little is known about the structure of gravity currents under conditions pertinent to building fires. The present investigation is an attempt to address this shortcoming by studying the turbulent structure of gravity currents. For this purpose, a series of experiments was conducted in a rectangular tank with turbulent, sub-critical underflows. Laser-Doppler Velocimetry was employed to quantify the velocity field and associated turbulent flow parameters. Experimental results indicated that the mean flow within the head region primarily consisted of an undiluted large single vortex which rapidly mixed with the ambient flow in the wake region. Cases with isothermal wall boundary conditions showed three-dimensional effects whereas those with adiabatic walls exhibited two-dimensional behaviour. Turbulence was found to be highly heterogeneous and its distribution was governed by the location of large eddies. While all components of turbulence kinetic energy showed minima in the regions where velocity was maximum (i.e. low fluid shear), they reached their maximum in the shear layer at the upper boundary of the flow. KEY WORDS: gravity currents, laser Doppler velocimetry, turbulent structure INTRODUCTION Gravity currents are important physical phenomena which have direct implications in a wide variety of physical situations ranging from environmental phenomena‡ to enclosure fires. -

Basic Thermodynamics-17ME33.Pdf
Module -1 Fundamental Concepts & Definitions & Work and Heat MODULE 1 Fundamental Concepts & Definitions Thermodynamics definition and scope, Microscopic and Macroscopic approaches. Some practical applications of engineering thermodynamic Systems, Characteristics of system boundary and control surface, examples. Thermodynamic properties; definition and units, intensive and extensive properties. Thermodynamic state, state point, state diagram, path and process, quasi-static process, cyclic and non-cyclic processes. Thermodynamic equilibrium; definition, mechanical equilibrium; diathermic wall, thermal equilibrium, chemical equilibrium, Zeroth law of thermodynamics, Temperature; concepts, scales, fixed points and measurements. Work and Heat Mechanics, definition of work and its limitations. Thermodynamic definition of work; examples, sign convention. Displacement work; as a part of a system boundary, as a whole of a system boundary, expressions for displacement work in various processes through p-v diagrams. Shaft work; Electrical work. Other types of work. Heat; definition, units and sign convention. 10 Hours 1st Hour Brain storming session on subject topics Thermodynamics definition and scope, Microscopic and Macroscopic approaches. Some practical applications of engineering thermodynamic Systems 2nd Hour Characteristics of system boundary and control surface, examples. Thermodynamic properties; definition and units, intensive and extensive properties. 3rd Hour Thermodynamic state, state point, state diagram, path and process, quasi-static