Butterfly Families (Appendix C from the Guidelines) (Pdf)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Lepidoptera: Papilionoidea) Q ⇑ Marianne Espeland A,B, , Jason P.W
Molecular Phylogenetics and Evolution 93 (2015) 296–306 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Ancient Neotropical origin and recent recolonisation: Phylogeny, biogeography and diversification of the Riodinidae (Lepidoptera: Papilionoidea) q ⇑ Marianne Espeland a,b, , Jason P.W. Hall c, Philip J. DeVries d, David C. Lees e, Mark Cornwall a, Yu-Feng Hsu f, Li-Wei Wu g, Dana L. Campbell a,h, Gerard Talavera a,i,j, Roger Vila i, Shayla Salzman a, Sophie Ruehr k, David J. Lohman l, Naomi E. Pierce a a Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, USA b McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Powell Hall, 2315 Hull Road, Gainesville, FL 32611, USA c Department of Systematic Biology-Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560-127, USA d Department of Biological Sciences, University of New Orleans, 2000 Lake Shore Drive, New Orleans, LA 70148, USA e Department of Zoology, University of Cambridge, Cambridge CB2 3EJ, UK f Department of Life Science, National Taiwan Normal University, Taipei, Taiwan g The Experimental Forest, College of Bio-Resources and Agriculture, National Taiwan University, Nantou, Taiwan h Division of Biological Sciences, School of Science, Technology, Engineering & Mathematics, University of Washington Bothell, Box 358500, 18115 Campus Way NE, Bothell, WA 98011-8246, USA i Institut de Biologia Evolutiva (CSIC-UPF), Pg. Marítim de la Barceloneta 37, 08003 Barcelona, Spain j Faculty of Biology & Soil Science, St. -

Self-Repair and Self-Cleaning of the Lepidopteran Proboscis
Clemson University TigerPrints All Dissertations Dissertations 8-2019 Self-Repair and Self-Cleaning of the Lepidopteran Proboscis Suellen Floyd Pometto Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Recommended Citation Pometto, Suellen Floyd, "Self-Repair and Self-Cleaning of the Lepidopteran Proboscis" (2019). All Dissertations. 2452. https://tigerprints.clemson.edu/all_dissertations/2452 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. SELF-REPAIR AND SELF-CLEANING OF THE LEPIDOPTERAN PROBOSCIS A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy ENTOMOLOGY by Suellen Floyd Pometto August 2019 Accepted by: Dr. Peter H. Adler, Major Advisor and Committee Co-Chair Dr. Eric Benson, Committee Co-Chair Dr. Richard Blob Dr. Patrick Gerard i ABSTRACT The proboscis of butterflies and moths is a key innovation contributing to the high diversity of the order Lepidoptera. In addition to taking nectar from angiosperm sources, many species take up fluids from overripe or sound fruit, plant sap, animal dung, and moist soil. The proboscis is assembled after eclosion of the adult from the pupa by linking together two elongate galeae to form one tube with a single food canal. How do lepidopterans maintain the integrity and function of the proboscis while foraging from various substrates? The research questions included whether lepidopteran species are capable of total self- repair, how widespread the capability of self-repair is within the order, and whether the repaired proboscis is functional. -

SYSTEMATICS of the MEGADIVERSE SUPERFAMILY GELECHIOIDEA (INSECTA: LEPIDOPTEA) DISSERTATION Presented in Partial Fulfillment of T
SYSTEMATICS OF THE MEGADIVERSE SUPERFAMILY GELECHIOIDEA (INSECTA: LEPIDOPTEA) DISSERTATION Presented in Partial Fulfillment of the Requirements for The Degree of Doctor of Philosophy in the Graduate School of The Ohio State University By Sibyl Rae Bucheli, M.S. ***** The Ohio State University 2005 Dissertation Committee: Approved by Dr. John W. Wenzel, Advisor Dr. Daniel Herms Dr. Hans Klompen _________________________________ Dr. Steven C. Passoa Advisor Graduate Program in Entomology ABSTRACT The phylogenetics, systematics, taxonomy, and biology of Gelechioidea (Insecta: Lepidoptera) are investigated. This superfamily is probably the second largest in all of Lepidoptera, and it remains one of the least well known. Taxonomy of Gelechioidea has been unstable historically, and definitions vary at the family and subfamily levels. In Chapters Two and Three, I review the taxonomy of Gelechioidea and characters that have been important, with attention to what characters or terms were used by different authors. I revise the coding of characters that are already in the literature, and provide new data as well. Chapter Four provides the first phylogenetic analysis of Gelechioidea to include molecular data. I combine novel DNA sequence data from Cytochrome oxidase I and II with morphological matrices for exemplar species. The results challenge current concepts of Gelechioidea, suggesting that traditional morphological characters that have united taxa may not be homologous structures and are in need of further investigation. Resolution of this problem will require more detailed analysis and more thorough characterization of certain lineages. To begin this task, I conduct in Chapter Five an in- depth study of morphological evolution, host-plant selection, and geographical distribution of a medium-sized genus Depressaria Haworth (Depressariinae), larvae of ii which generally feed on plants in the families Asteraceae and Apiaceae. -

E-News Winter 2019/2020
Winter e-newsletter December 2019 Photos Merry Christmas and a Happy New Year! INSIDE THIS ISSUE: Contributions to our newsletters Dates for your Diary & Winter Workparties....2 Borage - Painted Lady foodplant…11-12 are always welcome. Scottish Entomological Gathering 2020 .......3-4 Lunar Yellow Underwing…………….13 Please use the contact details Obituary - David Barbour…………..………….5 Chequered Skipper Survey 2020…..14 below to get in touch! The Bog Squad…………………………………6 If you do not wish to receive our Helping Hands for Butterflies………………….7 newsletter in the future, simply Munching Caterpillars in Scotland………..…..8 reply to this message with the Books for Sale………………………...………..9 word ’unsubscribe’ in the title - thank you. RIC Project Officer - Job Vacancy……………9 Coul Links Update……………………………..10 VC Moth Recorder required for Caithness….10 Contact Details: Butterfly Conservation Scotland t: 01786 447753 Balallan House e: [email protected] Allan Park w: www.butterfly-conservation.org/scotland Stirling FK8 2QG Dates for your Diary Scottish Recorders’ Gathering - Saturday, 14th March 2020 For everyone interested in recording butterflies and moths, our Scottish Recorders’ Gathering will be held at the Battleby Conference Centre, by Perth on Saturday, 14th March 2020. It is an opportunity to meet up with others, hear all the latest butterfly and moth news and gear up for the season to come! All welcome - more details will follow in the New Year! Highland Branch AGM - Saturday, 18th April 2020 Our Highlands & Island Branch will be holding their AGM on Saturday, 18th April in a new venue, Green Drive Hall, 36 Green Drive, Inverness, IV2 4EU. More details will follow on the website in due course. -

Washington Butterfly Association Common Butterflies of the Puget Sound Region and Their Food Plants
Washington Butterfly Association [email protected] Pine White ( Neophasia menapia ) w ww.naba.org/chapters/nabaws / Identification : White with black forewing patch, black veins below. Flight Period : late June – early October, peak in August. Common Butterflies of the Puget Sound Region Favorite Nectar Plants : Goldenrod, and Their Food Plants - By David Droppers Pearly Everlasting, Asters, Thistles. Larval Host Plants : Ponderosa Pine, Western Tiger Swallowtail ( Papilio Lodgepole Pine, Douglas-Fir, among other conifers. rutulus ). Identification : Large. Yellow with black tiger stripes. Cabbage White ( Pieris rapae) Underside with some blue. Flight Identification : White, black wing tips and Period : mid April – late September, spots. Males have one spot, females two peak in June. Favorite Nectar Plants : spots. Flight Period : early March – early Mock Orange, Milkweeds, Thistles, November, peaks in May, July and large showy flowers. Larval Host September. Favorite Nectar Plants: Plants : Native Willows, Quaking Many, especially garden flowers, such as Oregano and Lavender. Aspen and other poplars, Red Alder Larval Host Plants : Garden Brassicae, especially broccoli and cabbage. Anise Swallowtail ( Papilio zelicaon) Identification : Large. Mostly black, Cedar Hairstreak ( Mitoura grynea ) centrally yellow, with row of blue dots Identification : Small. Varying brown above, below buff brown on hindwing. Flight Period : late with violet tint, variable white postmedian line, small tails on March – late September, peaks in May, hindwings. Flight Period : late March – early August, peaks in July-August. Favorite Nectar Plants : May-June. Favorite Nectar Plants : Goldenrods, Yarrow, Many flowers, mostly large and showy. Dandelion, Clovers, Red Flowering Currant. Larval Host Plants : Garden Parsley Larval Host Plants : Western Red Cedar, Incense Cedar and Dill, Angelica, Cow Parsnip, many others. -

Big Creek Lepidoptera Checklist
Big Creek Lepidoptera Checklist Prepared by J.A. Powell, Essig Museum of Entomology, UC Berkeley. For a description of the Big Creek Lepidoptera Survey, see Powell, J.A. Big Creek Reserve Lepidoptera Survey: Recovery of Populations after the 1985 Rat Creek Fire. In Views of a Coastal Wilderness: 20 Years of Research at Big Creek Reserve. (copies available at the reserve). family genus species subspecies author Acrolepiidae Acrolepiopsis californica Gaedicke Adelidae Adela flammeusella Chambers Adelidae Adela punctiferella Walsingham Adelidae Adela septentrionella Walsingham Adelidae Adela trigrapha Zeller Alucitidae Alucita hexadactyla Linnaeus Arctiidae Apantesis ornata (Packard) Arctiidae Apantesis proxima (Guerin-Meneville) Arctiidae Arachnis picta Packard Arctiidae Cisthene deserta (Felder) Arctiidae Cisthene faustinula (Boisduval) Arctiidae Cisthene liberomacula (Dyar) Arctiidae Gnophaela latipennis (Boisduval) Arctiidae Hemihyalea edwardsii (Packard) Arctiidae Lophocampa maculata Harris Arctiidae Lycomorpha grotei (Packard) Arctiidae Spilosoma vagans (Boisduval) Arctiidae Spilosoma vestalis Packard Argyresthiidae Argyresthia cupressella Walsingham Argyresthiidae Argyresthia franciscella Busck Argyresthiidae Argyresthia sp. (gray) Blastobasidae ?genus Blastobasidae Blastobasis ?glandulella (Riley) Blastobasidae Holcocera (sp.1) Blastobasidae Holcocera (sp.2) Blastobasidae Holcocera (sp.3) Blastobasidae Holcocera (sp.4) Blastobasidae Holcocera (sp.5) Blastobasidae Holcocera (sp.6) Blastobasidae Holcocera gigantella (Chambers) Blastobasidae -

Pollinators Full.Pdf
Hymenoptera: Bees Hymenoptera: Bees Hymenoptera: Wasps, Ants & Sawies Hymenoptera: Wasps, Ants & Sawies Pollinator Insects of the South West Slopes of NSW and North East Victoria This guide has been prepared to aid identication of a Pollinator Insects selection of common pollinator insects. Insects Pollinator This guide provides a good starting point, but many species can look similar. Please see the references and websites listed if you would like help with accurate of the South West Slopes of NSW species identification. and North East Victoria An identification and conservation guide Halictid bee Hylaeus bee Gasteruptiid wasp Hairy ower wasp Halictidae Colletidae Gasteruptiidae Scoliidae of the South West Slopes NSW and North East Victoria Blue-banded bee Chequered cuckoo bee Ant Cream-spotted ichneumon wasp Apidae Apidae Formicidae Ichnuemonidae Hylaeus bee (bubbling) Large Lasioglossum sp. Orange ichneumon wasp Paper wasp Colletidae Halictidae Ichnuemonidae Vespidae Orange ichneumon wasp Ichnuemonidae Online pollinator information resources Aussie Bee aussiebee.com.au Bee Aware Australia beeawareaustralia.org Common spring bee European honey bee Cuckoo wasp European wasp Australian Museum Plant2pollinator Colletidae Apidae Chrysididae Vespidae australianmuseum.net.au/welcome-to-plant2pollinator PaDIL Australian Pollinators padil.gov.au/pollinators Bowerbird bowerbird.org.au Leafcutter bee Red bee Paper wasp Sawy adult Victorian butteries Megachilidae Halictidae Vespidae Tenthredinidae museumvictoria.com.au/bioinformatics/butter/images/bthumbmenu.htm Atlas of Living Australia ala.org.au Hymenoptera: Bees Hymenoptera: Wasps, Ants & Sawies Wild Pollinator Count wildpollinatorcount.com • Around 2,000 native bee species currently known. • Around 8,000 native species currently known; many more undescribed. Photography • Mostly found in sunny, open woodlands, gardens and meadows with lots • Found in all habitats. -

Genomic Analysis of the Tribe Emesidini (Lepidoptera: Riodinidae)
Zootaxa 4668 (4): 475–488 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2019 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4668.4.2 http://zoobank.org/urn:lsid:zoobank.org:pub:211AFB6A-8C0A-4AB2-8CF6-981E12C24934 Genomic analysis of the tribe Emesidini (Lepidoptera: Riodinidae) JING ZHANG1, JINHUI SHEN1, QIAN CONG1,2 & NICK V. GRISHIN1,3 1Departments of Biophysics and Biochemistry, University of Texas Southwestern Medical Center, and 3Howard Hughes Medical Insti- tute, 5323 Harry Hines Blvd, Dallas, TX, USA 75390-9050; [email protected] 2present address: Institute for Protein Design and Department of Biochemistry, University of Washington, 1959 NE Pacific Street, HSB J-405, Seattle, WA, USA 98195; [email protected] Abstract We obtained and phylogenetically analyzed whole genome shotgun sequences of nearly all species from the tribe Emesidini Seraphim, Freitas & Kaminski, 2018 (Riodinidae) and representatives from other Riodinidae tribes. We see that the recently proposed genera Neoapodemia Trujano, 2018 and Plesioarida Trujano & García, 2018 are closely allied with Apodemia C. & R. Felder, [1865] and are better viewed as its subgenera, new status. Overall, Emesis Fabricius, 1807 and Apodemia (even after inclusion of the two subgenera) are so phylogenetically close that several species have been previously swapped between these two genera. New combinations are: Apodemia (Neoapodemia) zela (Butler, 1870), Apodemia (Neoapodemia) ares (Edwards, 1882), and Apodemia (Neoapodemia) arnacis (Stichel, 1928) (not Emesis); and Emesis phyciodoides (Barnes & Benjamin, 1924) (not Apodemia), assigned to each genus by their monophyly in genomic trees with the type species (TS) of the genus. -
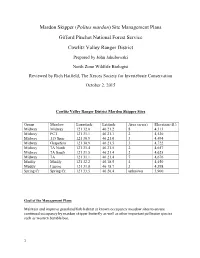
Mardon Skipper Site Management Plans
Mardon Skipper (Polites mardon) Site Management Plans Gifford Pinchot National Forest Service Cowlitz Valley Ranger District Prepared by John Jakubowski North Zone Wildlife Biologist Reviewed by Rich Hatfield, The Xerces Society for Invertebrate Conservation October 2, 2015 Cowlitz Valley Ranger District Mardon Skipper Sites Group Meadow Longitude Latitude Area (acres) Elevation (ft.) Midway Midway 121 32.0 46 21.2 8 4,313 Midway PCT 121 31.1 46 21.1 2 4,530 Midway 115 Spur 121 30.9 46 21.0 3 4,494 Midway Grapefern 121 30.9 46 21.5 3 4,722 Midway 7A North 121 31.4 46 21.5 2 4,657 Midway 7A South 121 31.5 46 21.4 2 4,625 Midway 7A 121 31.1 46 21.4 7 4,676 Muddy Muddy 121 32.2 46.18.5 4 4,450 Muddy Lupine 121 31.8 46 18.7 3 4,398 Spring Cr Spring Cr. 121 33.5 46 20.4 unknown 3,900 Goal of the Management Plans Maintain and improve grassland/forb habitat at known occupancy meadow sites to ensure continued occupancy by mardon skipper butterfly as well as other important pollinator species such as western bumble bee. 1 Introduction On the Gifford Pinchot National Forest (GPNF), mardon skippers were first detected on the Mt. Adams Ranger District (MTA) in 2000 and on Cowlitz Valley Ranger District (CVRD) in 2002. Mardon skippers are known to inhabit ten, upland dry grassy meadows on the CVRD. Portions of the meadows are mesic and are unsuitable mardon skipper habitat. -

Butterflies from the Middle Eocene: the Earliest Occurrence of Fossil Papilionoidea (Lepidoptera)
THE PEARCE- SELLARDS Sctks NUMBER 29 BUTTERFLIES FROM THE MIDDLE EOCENE: THE EARLIEST OCCURRENCE OF FOSSIL PAPILIONOIDEA (LEPIDOPTERA) Christopher J. Durden and Hugh Rose 1978 Texas Memorial Museum/2400 Trinity/Austin, Texas 78705 W. W. Newcomb, Director The Pearce-Sellards Series is an occasional, miscellaneous series of brief reports of museum and museum associated field investigations and other research. Its title seeks to commemorate the first two directors of the Texas Memorial Museum, now both deceased: J. E. Pearce and Dr. E. H. Sellards, professors of anthropology and geology respectively, of The University of Texas. A complete list of Pearce-Sellards papers, as well as other publica- tions of the museum, will be sent upon request. BUTTERFLIES FROM THE MIDDLE EOCENE: THE EARLIEST OCCURRENCE OF FOSSIL PAPILIONOIDEA (LEPIDOPTERA) 1 Christopher J. Durden 2 and Hugh Rose 3 ABSTRACT Three fossil butterflies recently collected from the Green River Shale of Colorado extend the known range of Rhopalocera eight to ten million years back, to 48 Ma. Praepapilio Colorado n. g., n. sp., and P. gracilis n. sp. are primitive Papilionidae related to the modern Baronia brevicornis Salvin, but they require a new subfamily, Praepapilioninae. Riodinella nympha n. g., n. sp. is a primitive member of the Lycaenidae, related to modern Ancyluris, Riodina, and Rhetus, in the tribe Riodinidi. INTRODUCTION With approximately 194,000 living species, the Lepidoptera is, after the Coleoptera with some 350,000, species, the second most diverse order of organisms. It is underrepresented in the fossil record (Scudder 1875, 1891, 1892; Handlirsch 1925;Mackay 1970;Kuhne 1973; Shields 1976). -

Lepidoptera, Nymphalidae, Biblidinae) and Patterns of Morphological Similarity Among Species from Eight Tribes of Nymphalidae
Revista Brasileira de Entomologia http://dx.doi.org/10.1590/S0085-56262013005000006 External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae Luis Anderson Ribeiro Leite1,2, Mirna Martins Casagrande1,3 & Olaf Hermann Hendrik Mielke1,4 1Departamento de Zoologia, Setor de Ciências Biológicas, Universidade Federal do Paraná, Caixa Postal 19020, 81531–980 Curitiba-PR, Brasil. [email protected], [email protected], [email protected] ABSTRACT. External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae. The external structure of the integument of Dynamine postverta postverta (Cramer, 1779) is based on detailed morphological drawings and scanning electron microscopy. The data are compared with other species belonging to eight tribes of Nymphalidae, to assist future studies on the taxonomy and systematics of Neotropical Biblidinae. KEYWORDS. Abdomen; head; Insecta; morphology; Papilionoidea; thorax. Nymphalidae is a large cosmopolitan family of butter- served in dorsal view (Figs. 1–4). Two subspecies are recog- flies, with about 7,200 described species (Freitas & Brown nized according to Lamas (2004), Dynamine postverta Jr. 2004) and is perhaps the most well documented biologi- postverta (Cramer, 1779) distributed in South America and cally (Harvey 1991; Freitas & Brown Jr. 2004; Wahlberg et Dynamine postverta mexicana d’Almeida, 1952 with a dis- al. 2005). The systematic relationships are still somewhat tribution restricted to Central America. Several species sur- unclear with respect to its subfamilies, tribes and genera, and veys and other studies cite this species as Dynamine mylitta even after more than a century of studies on these groups, (DeVries 1987; Mielke 1994; Miller et al.1999; Freitas & these relationships still seem to confuse many who set out to Brown, Jr. -

Recent Diversification of Chrysoritis Butterflies in the South African Cape
Molecular Phylogenetics and Evolution 148 (2020) 106817 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Recent diversification of Chrysoritis butterflies in the South African Cape (Lepidoptera: Lycaenidae) T ⁎ ⁎ Gerard Talaveraa,b, ,Zofia A. Kaliszewskab,c, Alan Heathb,d, Naomi E. Pierceb, a Institut de Biologia Evolutiva (CSIC-UPF), Passeig Marítim de la Barceloneta 37, 08003 Barcelona, Catalonia, Spain b Department of Organismic and Evolutionary Biology and Museum of Comparative Zoology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, United States c Department of Biology, University of Washington, Seattle, WA 98195, United States d Iziko South African Museum, Cape Town, South Africa ARTICLE INFO ABSTRACT Keywords: Although best known for its extraordinary radiations of endemic plant species, the South African fynbos is home Butterflies to a great diversity of phytophagous insects, including butterflies in the genus Chrysoritis (Lepidoptera: Chrysoritis Lycaenidae). These butterflies are remarkably uniform morphologically; nevertheless, they comprise 43 cur- Fynbos rently accepted species and 68 currently valid taxonomic names. While many species have highly restricted, dot- Phylogeny like distributions, others are widespread. Here, we investigate the phylogenetic and biogeographic history un- Radiation derlying their diversification by analyzing molecular markers from 406 representatives of all described species Speciation Taxonomy throughout their respective ranges. We recover monophyletic clades for both C. chrysaor and C. thysbe species- groups, and identify a set of lineages that fall between them. The estimated age of divergence for the genus is 32 Mya, and we document significantly rapid diversification of the thysbe species-group in the Pleistocene (~2 Mya).