152 Bashir CORRECTED
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Final Report
-, FEDERAL REPUBLIC OF NIGERIA RURAL ACCESS AND MOBILITY PROJECT (RAMP) FINAL REPORT CONSULTANCY SERVICES FOR STUDY TO PRIORITIZE INTERVENTION AREAS IN KADUNA STATE - 1AND TO SELECT THE INITIAL ROAD PROGRAM IN SUPPORT OF SUCH PRIORITIZED AREAS STATE COORDINATING OFFICE: - NATIONAL COORDINATING OFFICE: Federal Project Management Unit (FPMU) State Project Implementation Unit (SPIU) 'Federal Department of Rural Development C/O State Ministry of Works & Transport Kaduna. - NAIC House, Plot 590, Zone AO, Airport Road Central Area, Abuja. 3O Q5 L Tel: 234-09-2349134 Fax: 234-09-2340802 CONSULTANT:. -~L Ark Consult Ltd Ark Suites, 4th Floor, NIDB House 18 Muhammadu Buhari Way Kaduna.p +Q q Tel: 062-2 14868, 08033206358 E-mail: [email protected] TABLE OF CONTENTS EXECUTIVE SUMMARY Introduction 1 Scope and Procedures of the Study 1 Deliverables of the Study 1 Methodology 2 Outcome of the Study 2 Conclusion 5 CHAPTER 1: PREAMBLE 1.0 Introduction 6 1.1 About Ark Consult 6 1.2 The Rural Access and Mobility Project (RAMP) 7 1.3 Terms of Reference 10 1.3.1 Scope of Consultancy Services 10 1.3.2 Criteria for Prioritization of Intervention Areas 13 1.4 About the Report 13 CHAPTER 2: KADUNA STATE 2.0 Brief About Kaduna State 15 2.1 The Kaduna State Economic Empowerment and Development Strategy 34 (KADSEEDS) 2.1.1 Roads Development 35 2.1.2 Rural and Community Development 36 2.1.3 Administrative Structure for Roads Development & Maintenance 36 CHAPTER 3: IDENTIFICATION & PRIORITIZATION OF INTERVENTION AREAS 3.0 Introduction 40 3.1 Approach to Studies 40 -
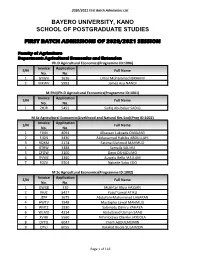
First Batch Admissions of 2020/2021 Session
2020/2021 First Batch Admissions List BAYERO UNIVERSITY, KANO SCHOOL OF POSTGRADUATE STUDIES FIRST BATCH ADMISSIONS OF 2020/2021 SESSION Faculty of Agriculture Department: Agricultural Economics and Extension Ph.D Agricultural Economics(Programme ID:1006) Invoice Application S/N Full Name No. No. 1 GYWH 1636 Umar Muhammad IBRAHIM 2 MXWV 5993 James Asu NANDI M.Phil/Ph.D Agricultural Economics(Programme ID:1001) Invoice Application S/N Full Name No. No. 1 ZHJR 5491 Sadiq Abubakar SADIQ M.Sc Agricultural Economics(Livelihood and Natural Res Eco)(Prog ID:1002) Invoice Application S/N Full Name No. No. 1 FJVN 4091 Alhassan Lukpada DANLAMI 2 FJQN 3430 Abdussamad Habibu ABDULLAHI 3 RQXM 2174 Fatima Mahmud MAHMUD 4 BTRW 3488 Samaila SALIHU 5 CFQW 3100 Dayo OSHADUMO 6 RVWZ 3360 Auwalu Bello MALLAM 7 FDZV 5504 Ngbede Sabo EDO M.Sc Agricultural Economics(Programme ID:1002) Invoice Application S/N Full Name No. No. 1 QWXB 330 Mukhtar Aliyu HASSAN 2 XHJC 5427 Yusuf Lawal ATIKU 3 JZFP 1675 Abdullahi Muhammad LABARAN 4 HWTV 1948 Mustapha Lawal MAHMUD 5 RMTZ 1930 Salamatu Dahiru YAHAYA 6 WLMD 4314 Abdulbasid Usman SAAD 7 XVHK 5560 Nihinlolawa Olanike JAYEOLA 8 DYTQ 6047 Imam ABDULMUMIN 9 CPVJ 6055 Barakat Bisola SULAIMON Page 1 of 116 2020/2021 First Batch Admissions List M.Sc Agricultural Extension(Programme ID:1003) Invoice Application S/N Full Name No. No. 1 DMWZ 36 Olayinka Adebola BELLO 2 PYHF 518 Isiagu Benedeth ANTHONY 3 RVFW 2291 Obako ENAHIBRAHIM 4 XFYN 2366 Kabiru MUSA 5 HBYD 5171 Musa GARBA Department: Agronomy Ph.D Agronomy(Programme ID:1108) Invoice Application S/N Full Name No. -

Sero-Prevalence Survey of Rubella Igm Antibodies Among Pregnant Women in Kano, Nigeria
International Journal of Life Science and Engineering Vol. 1, No. 2, 2015, pp. 56-60 http://www.aiscience.org/journal/ijlse Sero-Prevalence Survey of Rubella IgM Antibodies among Pregnant Women in Kano, Nigeria Yahaya H.*, Ibrahim A., Muhammad A. B., Dandawaki S. M. Department of Medical Laboratory Science, Faculty of Allied Health Sciences, Bayero University, Kano, Nigeria Abstract Rubella is a generally mild illness and its serious complications are rare. Although a major section of pregnant women are immune, cases of rubella infection occur in Nigeria among pregnant women. This study was aimed at assessing the prevalence of rubella virus IgM antibody among obstetric population between 15 – 44 age group attending Antenatal Clinic Bamalli Nuhu Maternity Hospital, Kano. The samples were analyses using Enzyme-Linked Immunosorbent Assay technique. A well- structured questionnaire was administered to the subjects to obtain socio-demographic data. Of the total 89 samples screened, 7 (7.87%) were reactive to rubella IgM antigens from the coated wells. Out of 7 positive samples, 4 (4.49%) were within 15–24 years age group, representing the most susceptible group, while the infection rate was lowest among 35–44 age group with prevalence rate of 1 (1.12%). Furthermore, women in the third trimesters of pregnancy were recorded to have the highest prevalence rate to the antibody 3(3.37%) while those at first and second trimesters recorded 1(1.12%) and 2(2.25%) respectively. In addition, no case was recorded among women with higher educational background recorded as compared to those who did not acquire western education with prevalence of 4(4.49%). -

NDA AFSB List
CONFIDENTIAL NIGERIAN DEFENCE ACADEMY ARMED FORCES SELECTION BOARD FOR THE 72ND REGULAR COURSE 1. The Nigerian Defence Academy Screening Test held on Saturday 15 August 2020 result is out. Candidates who sat for the Screening Test should log onto www.nda.edu.ng or www.ndaapplications.net to check for their names. Shortlisted candidates are to appear for interview before the Armed Forces Selection Board (AFSB) from Saturday 12 September - 24 October 2020 at the Nigerian Defence Academy Ribadu Campus, Kaduna. Candidates will appear before the AFSB in 3 Batches as follows: a. Batch 1. Candidates from the underlisted are toreportto NDA on Saturday 12 September 2020. (1) Anambra (2) Bauchi ( (3) Delta (4) Edo (5) Ekiti (6) Enugu (7) Gombe (8) Kano (9) Kaduna (10) Nasarawa (11) Ondo (12) Plateau b. Batch 2. Candidates from the underlisted States are to report to NDA on Saturday 26 September 2020 (1) Abia (2) Adamawa (3) Akwa Ibom (4) Benue (5) Imo (6) Katsina (7) Kogi (8) Osun (9) Oyo (10) Rivers (11) Yobe (12) Zamfara c. Batch 3. Candidates from the underlisted States are to report to NDA on Saturday 10 October 2020. (1) Bayelsa (2) Borno (3) Cross River (4) Ebonyi (5) FCT (6) Jigawa 1 CONFIDENTIAL CONFIDENTIAL (7) Kebbi (8) Kwara (9) Lagos (10) Niger (11) Ogun (12) Sokoto (13) Taraba 2. Candidates who fail to report on Saturday 12 September 2020, Saturday 26 September 2020 and Saturday 10 October 2020 for Batches 1, 2 and 3 respectively, will not be allowed to attend the interview. Any candidate in Batches 2 and 3 who is seen around the NDA premises before Saturday 26 September 2020 and Saturday 10 October 2020 respectively, will be disqualified. -

The Association of Radiographers of Nigeria, Book of Abstracts, 2018
0 The Association of Radiographers of Nigeria, Book of Abstracts, 2018 NATIONAL EXECUTIVE OFFICERS OF THE ARN (2017 – 2019) 1. Patron- - - - - Senator, Maj Gen (Retd) Ike OS Nwachukwu (CFR, Mni, FSS, DSS) 2. President- - - - - Ola Elizabeth Balogun, Mrs (M.Sc, FARN) 3. Vice President I - - - Sani T. Kabir (DIR) 4. Vice President II - - - Tom Adejoh (Ph.D) 5. National Secretary- - - Musa Y. Dambele (M.Sc) 6. Assistant Nat Sec I - - Samaila A. Baba (M.Sc) 7. Assistant Nat Sec II - - Ibrahim Adegboye (DIR) 8. National Treasurer- - - Mabel C. Ugwuja, Mrs (M.Sc) 9. Financial Secretary- - - Samuel Shem Laushugno (M.Sc) 10. Public Relations Officer- - Joshua J. Sule (B.Sc) 11. Immediate Past President- - Mark C. Okeji (Ph.D, FARN) 12. Immediate Past Treasurer- - Imoh S. Udoh (M.Sc) 13. Registrar, RRBN - - - Mark C. Okeji (Ph.D, FARN) 1 The Association of Radiographers of Nigeria, Book of Abstracts, 2018 FOREWORD The present Council of ARN (2017 – 2019) led by Mrs Ola Elizabeth Balogun (M.Sc, FARN), was inaugurated in January 2017. Subsequently and swiftly, she constituted the education committee (EDUCOM), with the following members: 1. Adejoh, Tom (Ph.D) - NAUTH, Nnewi; Clinical Radiographer 2. Joseph, Z. Dlama (Ph.D) – BUK, Kano; Academic 3. Okeji, C. Mark (Ph.D) – UNN, Enugu Campus/RRBN Abuja; Academic 4. Nkubli, B. Flavious (M.Sc) – UNIMAID, Maiduguri; Academic 5. Luntsi, Geofery (M.Sc) - UNIMAID, Maiduguri; Academic 6. Balogun-Adebiyi I. Rohimat (B.Sc) – NOHI, Lagos; Clinical Radiographer The EDUCOM, unlike those before it, was given tough and multiple terms of reference: determination of conference themes, coordination of conference papers, communique drafting, coordination of World Radiography Day (WRD) celebrations on 8th November and, to ensure the continuity of Journal of Radiography & Radiation Sciences (JRRS). -

The Venus Medicare Hospital Directory
NATIONWIDE PROVIDER DIRECTORY CATEGORY 1 HOSPITALS S/N STATE CITY/TOWN/DISTRICT HEALTHCARE PROVIDER ADDRESS TERMS & CONDITIONS SERVICES Primary, O&G, General Surgery, Paediatrics, 1 FCT/ABUJA CENTRAL AREA Limi Hospital & Maternity Plot 541Central District, Behind NDIC/ICPC HQ, Abuja Nil Internal Medicine Primary, Int. Med, Paediatrics,O&G, GARKI Garki Hospital Abuja Tafawa Balewa Way, Area 3, Garki, Abuja Private Wards not available. Surgery,Dermatology Amana Medical Centre 5 Ilorin str, off ogbomosho str,Area 8, Garki Nil No.5 Plot 802 Malumfashi Close, Off Emeka Anyaoku Strt, Alliance Clinics and Services Area 11 Nil Primary, Int. Med, Paediatrics Primary, O&G, General Surgery, Paediatrics, Internal Medicine Dara Medical Clinics Plot 202 Bacita Close, off Plateau Street, Area 2 Nil Primary, O&G, General Surgery, Paediatrics, Internal Medicine Sybron Medical Centre 25, Mungo Park Close, Area 11, Garki, Abuja Nil Primary, O&G, Paediatrics,Int Medicine, Gen Guinea Savannah Medical Centre Communal Centre, NNPC Housing Estate, Area II, Garki Nil Surgery, Laboratory Bio-Royal Hospital Ltd 11, Jebba Close, Off Ogun Street, Area 2, Garki Nil Primary, Surgery, Paediatrics. Primary, Family Med, O & G, Gen Surgery, Royal Specialist Hospital 2 Kukawa Street, Off Gimbiyan Street Area 11, Garki, Abuja Nil Urology, ENT, Radiology, Paediatrics No.4 Ikot Ekpene Close, Off Emeka Anyaoku Street Crystal Clinics (opposite National Assembly Clinic) Garki Area 11 Nil Primary, O&G, Paediatrics, Internal Medicine, Surgery Model Care Hospital & Consultancy Primary, Paediatrics, Surgery and ENT Limited No 5 Jaba Close, off Dunukofia Street by FCDA Garki, Abuja Nil Surgery Primary, O&G, General Surgery, Paediatrics, GARKI II Fereprod Medical Centre Obbo Crescent, off Ahmadu Bello Way, Garki II Nil Internal Medicine Holy Trinity Hospital 22 Oroago Str, by Ferma/Old CBN HQ, Garki II Nil Primary only. -

Resricted 1 Restricted Schedule of Activities For
RESRICTED SCHEDULE OF ACTIVITIES FOR THE 2020 NIGERIAN AIR FORCE AIRMEN/AIRWOMEN RECRUITMENT SELECTION BOARD INTERVIEW 1. The underlisted candidates are hereby invited to attend Year 2020 Nigerian Air Force Airmen/Airwomen Recruitment Selection Interview at the Nigerian Air Force Base, Kawo – Kaduna from 24 September - 12 November 2020. Candidates are to report with the following: a. Originals and Photocopies of their Credentials. b. 2 White vests. c. 2 Blue PT Shorts. d. One Pair of Canvas Shoes. e. 4 Passport Photographs. f. Face Masks. g. Hand Sanitizer. 2. The Candidates are required to report in Batches as indicated below: a. Batch A - Thursday 24 - 30 September 2020 (1) Abia (2) Benue (3) Ekiti (4) Kano (5) Nasarawa (6) Plateau b. Batch B - Thursday 1 – 7 October 2020 (1) Adamawa (2) Borno (3) Enugu 1 RESTRICTED RESRICTED (4) Katsina (5) Niger (6) Rivers c. Batch C - Thursday 8 – 14 October 2020 (1) Akwa Ibom (2) Cross River (3) Gombe (4) Kebbi (5) Ogun (6) Sokoto d. Batch D - Thursday 15 – 21 October 2020 (1) Anambra (2) Delta (3) Imo (4) Kogi (5) Ondo (6) Taraba e. Batch E - Thursday 22 – 28 October 2020 (1) Bauchi (2) Ebonyi (3) Jigawa (4) Kwara (5) Osun (6) Yobe f. Batch F - Thursday 29 October – 5 November 2020 (1) Bayelsa (2) Edo (3) Kaduna 2 RESTRICTED RESRICTED (4) Lagos (5) Oyo (6) Zamfara (7) FCT Note: Face masks are to be worn and other COVID-19 preventive measures are to be strictly observed throughout the exercise. SIGNED MAHMOUD EL-HAJI AHMED Air Vice Marshal for Chief of the Air Staff 3 RESTRICTED RESTRICTED SHORTLISTED -

Agulu Road, Adazi Ani, Anambra State. ANAMBRA 2 AB Microfinance Bank Limited National No
FINANCIAL POLICY AND REGULATION DEPARTMENT LICENSED MICROFINANCE BANKS (MFBs) IN NIGERIA AS AT MAY 25, 2016 # Name Category Address State Description 1 AACB Microfinance Bank Limited State Nnewi/ Agulu Road, Adazi Ani, Anambra State. ANAMBRA 2 AB Microfinance Bank Limited National No. 9 Oba Akran Avenue, Ikeja Lagos State. LAGOS 3 Abatete Microfinance Bank Limited Unit Abatete Town, Idemili Local Govt Area, Anambra State ANAMBRA 4 ABC Microfinance Bank Limited Unit Mission Road, Okada, Edo State EDO 5 Abia State University Microfinance Bank Limited Unit Uturu, Isuikwuato LGA, Abia State ABIA 6 Abigi Microfinance Bank Limited Unit 28, Moborode Odofin Street, Ijebu Waterside, Ogun State OGUN 7 Abokie Microfinance Bank Limited Unit Plot 2, Murtala Mohammed Square, By Independence Way, Kaduna State. KADUNA 8 Abucoop Microfinance Bank Limited State Plot 251, Millenium Builder's Plaza, Hebert Macaulay Way, Central Business District, Garki, Abuja FCT 9 Accion Microfinance Bank Limited National 4th Floor, Elizade Plaza, 322A, Ikorodu Road, Beside LASU Mini Campus, Anthony, Lagos LAGOS 10 ACE Microfinance Bank Limited Unit 3, Daniel Aliyu Street, Kwali, Abuja FCT 11 Acheajebwa Microfinance Bank Limited Unit Sarkin Pawa Town, Muya L.G.A Niger State NIGER 12 Achina Microfinance Bank Limited Unit Achina Aguata LGA, Anambra State ANAMBRA 13 Active Point Microfinance Bank Limited State 18A Nkemba Street, Uyo, Akwa Ibom State AKWA IBOM 14 Acuity Microfinance Bank Limited Unit 167, Adeniji Adele Road, Lagos LAGOS 15 Ada Microfinance Bank Limited Unit Agwada Town, Kokona Local Govt. Area, Nasarawa State NASSARAWA 16 Adaigbo Microfinance Bank Limited Unit 12, NEPA Road, Ogwashi-Uku, Delta State. -

Adherence to Anti Patients Attending P Dherence To
Special Conference Edition , November, 2019 http://dx.doi.org/10.4314/bajopas.v12i1.59S Bayero Journal of Pure and Applied Sciences, 12(1): 395 - 400 ISSN 2006 – 6996 ADHERENCE TO ANTIHYPERTENSIVE MEDICATIONS IN PATIENTS ATTENDING PUBLIC HOSPITALS IN KANO STATE , NIGERIA 1, 2* Umar Idris Ibrahim, Shafiu Mohammed 2, Abdulkadir Umar Zezi 3 and 4Basira Kankia Lawal 1. Department of Clinical Pharmacy and Pharmacy PractiPracticece,, Faculty of Pharmaceutical Sciences, Bayero University Kano, Nigeria. 2. Department of Clinical Pharmacy and Pharmacy PractiPracticece,, Faculty of Pharmaceutical Sciences, Ahmadu Bello University Zaria, Nigeria. 3. Department of Pharmacology and Therapeutics, FacultFacultyy of Pharmaceutical Sciences, Ahmadu Bello University Zaria, Nigeria. 4. Department of Clinical Pharmacy and Pharmacy ManagemManagement,ent, Faculty of Pharmaceutical Sciences, Kaduna State University, Nigeria. Correspondence author: [email protected] +2348038850147 ABSTRACT Hypertension is a chronic medical condition characterized by an elevated arterial blood pressure with increasing prevalence in developing countries including Nigeria. One of the integral elements in management of hypertension is adherence to medication and life- style modification. This study aimed to assess adherence level for anti -hypertensive medications among adult hypertensive patients attending public hospitals in Kano State, Nigeria. The study was a cross sectional prospective survey involving 600 pat ients from six public healthcare facilities selected by multistage sampling technique. Adherence status was assessed using Morisky medication adherence scale. Sociodemographic data and other factors that may influence adherence to hypertension medications were evaluated. Out of the 598 patients that participated in the study, only 178 (29.8%) have their BP controlled based on JNC8. Three hundred and thirty two (55.5%) out of 598 patients have good adherence, while 266 (45.5%) have poor adherence. -

Yinka O Lom Ojobi
EXPLANING THE DYNAMICS OF ISLAM AND CONFLICT: THE CASE OF NORTHERN NIGERIA Yinka Olomojobi ProQuest Number: 11003694 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 11003694 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 EXPLANING THE DYNAMICS OF ISLAM AND CONFLICT: THE CASE OF NORTHERN NIGERIA Yinka Olomojobi, LL.B (Hons), LL.M (Liverpool), Barrister & Solicitor, Supreme Court of Nigeria Submitted to the Department of Politics and International Relations, Lancaster University In fulfilment of the requirements for the degree of Doctor of Philosophy October 2010 2 I declare that the work presented in this thesis is the author’s own and has not been previously submitted for the award of a higher degree at any university Signed. 3 Abstract Nigeria has a complex ethno-religious profile. Thanks to the British colonial administration, a myriad of individual groups professing various faiths and belonging to different ethnicities have found themselves in a tense, unsettled and competitive political system. Unsurprisingly, there have been several attempts to undermine the profile of the state. -

Seroprevalence and Risk Factors of Cytomegalovirus and Rubella Virus Infections Amongst Pregnant Women Attending Some Hospitals in Maiduguri Metropolis, Nigeria
International Journal of Research e-ISSN: 2348-6848 p-ISSN: 2348-795X Available at https://pen2print.org/index.php/ijr/ Volume 05 Issue 20 September 2018 Seroprevalence and Risk Factors Of Cytomegalovirus and Rubella Virus Infections amongst Pregnant Women Attending Some Hospitals in Maiduguri Metropolis, Nigeria 1Fatima Maina Muhammad 2M. B. Abubakar 3A.D.EL-Yuguda *4A.A.G. Benisheikh Email:[email protected] 1. Sir Kashim Ibrahim College of Education, Maiduguri, Borno State 2. Department of Veterinary Microbiology, University of Maiduguri 3. Department of Veterinary Microbiology, University of Maiduguri 4. North East Zonal Biotechnology Center for Excellence, University of Maiduguri/University of Wolverhampton, United Kingdom Abstract This study was designed to determine the seroprevalence of CMV and Rubella virus infections in pregnant women attending antenatal clinics in Maiduguri Nigeria and to identify possible risk factors associated with the transmission and spread of the two diseases using ELISA tests and a structured questionnaire. A total number of 300 blood samples from pregnant women was collected to detect IgM and IgG antibodies against the two viruses .An overall CMV and Rubella virus seroprevalence of 40% and 18.7% respectively was observed among the pregnant women in the study area. Distribution of the positive samples in the different hospitals showed CMV has 14.3 % IgG at UMTH (University of Maiduguri Teaching Hospital) while Rubella virus IgG seroprevalence was 8.7% in state specialist hospital (p<0.05). Also, CMV has 2.3% IgM, 35.0% IgG and 3.0% mixed IgM and IgG antibody classes, while Rubella virus seroprevalence showed 8.3% IgM, 6.7% IgG and 3.7% mixed IgM and IgG antibody classes. -

2016 Annual Account A4
2016 ANNUAL REPORT & ACCOUNTS INSURANCE REGENCY ALLIANCE INSURANCE PLC RC 223946 We earn your trust www.regencyalliance.com Authorized and Regulated by the National Insurance Commission RIC 034 Table of Contents Consolidated and Separate Financial Statements INSURER for the year ended 31 December 2016 RC: 023048 Notice of Meeting...................................................................2 Corporate Information . ......................3 Financial Highlights . .......................4 Directors Certification . .........................5 Statement of Directors' Responsibilities . ...........................6 Chairman's Statement. .....................7 Board of Directors...................................................................9 Board Performance Evaluation . .....................14 Management Staff . ...................15 Report of the Directors . ......................16 Corporate Governance Report . ....................21 Management Discussion and Analysis . .....................25 Audit Committee Report . ....................26 Independent Auditors' Report . ......................27 Statement of Significant Accounting Policies . ...........................30 Statement of Financial Position. ........................53 Statement of Profit or Loss and other Comprehensive Income. ...........................54 Statement of Changes in Equity . ......................55 Statement of Cash Flows . ....................57 Notes to the Accounts . .....................58 Enterprise Risk Management . ....................84 Assets/Liability