Antimicrobial Activity of Bioactive Compounds of Haplopappus Multifolius and Haplopappus Taeda Against Human Pathogenic Microorganisms
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Notes on Variation and Geography in Rayjacksonia Phyllocephala (Asteraceae: Astereae)
Nesom, G.L., D.J. Rosen, and S.K. Lawrence. 2013. Notes on variation and geography in Rayjacksonia phyllocephala (Asteraceae: Astereae). Phytoneuron 2013-53: 1–15. Published 12 August 2013. ISSN 2153 733X NOTES ON VARIATION AND GEOGRAPHY IN RAYJACKSONIA PHYLLOCEPHALA (ASTERACEAE: ASTEREAE) GUY L. NESOM 2925 Hartwood Drive Fort Worth, Texas 76109 [email protected] DAVID J. ROSEN Department of Biology Lee College Baytown, Texas 77522-0818 [email protected] SHIRON K. LAWRENCE Department of Biology Lee College Baytown, Texas 77522-0818 ABSTRACT Inflorescences of Rayjacksonia phyllocephala in the disjunct Florida population system are characterized by heads on peduncles with leaves mostly reduced to linear bracts; heads in inflorescences of the Mexico-Texas-Louisiana system are immediately subtended by relatively unreduced leaves. The difference is consistent and justifies recognition of the Florida system as R. phyllocephala var. megacephala (Nash) D.B. Ward. Scattered waifs between the two systems are identified here as one or the other variety, directly implying their area of origin. In the eastern range of var. phyllocephala , at least from Brazoria County, Texas, eastward about 300 miles to central Louisiana, leaf margins vary from entire to deeply toothed-spinulose. In contrast, margins are invariably toothed-spinulose in var. megacephala as well as in the rest of southeastern Texas (from Brazoria County southwest) into Tamaulipas, Mexico. In some of the populations with variable leaf margins, 70-95% of the individuals have entire to mostly entire margins. KEY WORDS : Rayjacksonia , morphological variation, leaf margins, disjunction, waifs Rayjacksonia phyllocephala (DC.) Hartman & Lane (Gulf Coast camphor-daisy) is an abundant and conspicuous species of the shore vegetation around the Gulf of Mexico. -
1 C M 2 B H 3 B M 4 C M 5 B L 6 B M 7 B M 8
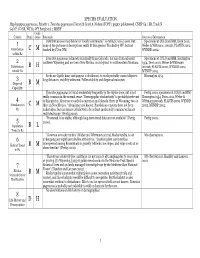
SPECIES EVALUATION Haplopappus pygmaeus, Priority 1. Tonestus pygmaeus (Torrey & Gray) A. Nelson (TOPY). pygmy goldenweed. CNHP G4 / SR, Track N G4 N?. CO SR, WY S1. WY Peripheral 2 MBNF Confi- Criteria Rank dence Rationale Sources of Information Distribution (see map below) is “nearly continuous,” so rating C was chosen, but Specimens at COLO and RM, Dorn 2001, 1 none of the pictures or descriptions really fit this species. Tracked by WY, but not Weber & Wittmann 2001ab, PLANTS 2002, Distribution C M tracked by CO or NM. WYNDD 2002. within R2 Tonestus pygmaeus is known principally from Colorado, but also from adjacent Specimens at COLO and RM, Harrington 2 southern Wyoming and northern New Mexico, and disjunct in southwestern Montana. 1954, Dorn 2001, Weber & Wittmann Distribution B H 2001ab, PLANTS 2002, WYNDD 2002, outside R2 MTNHP 2002. Seeds are lightly hairy and pappus is deciduous, so seeds probably cannot disperse Harrington 1954. 3 long distances; viability unknown. Pollen viability and dispersal unknown. Dispersal B M Capability Tonestus pygmaeus is found moderately frequently in the Alpine zone, but is not Fertig 2000, specimens at COLO and RM, really common in the normal sense. “Demographic stochasticity” is probably irrelevant Harrington 1954, Dorn 2001, Weber & 4 to this species. About 50 recorded occurrences in Colorado, three in Wyoming, two or Wittmann 2001ab, PLANTS 2002, WYNDD Abundance in C M three in New Mexico. “Abundance not known. Population censuses have not been 2002, MTNHP 2002. R2 undertaken, but the species is believed to be at least moderately common within its restricted range” (Fertig 2000). -

Reclassification of North American Haplopappus (Compositae: Astereae) Completed: Rayjacksonia Gen
AmericanJournal of Botany 83(3): 356-370. 1996. RECLASSIFICATION OF NORTH AMERICAN HAPLOPAPPUS (COMPOSITAE: ASTEREAE) COMPLETED: RAYJACKSONIA GEN. NOV.1 MEREDITH A. LANE2 AND RONALD L. HARTMAN R. L. McGregor Herbarium(University of Kansas NaturalHistory Museum Division of Botany) and Departmentof Botany,University of Kansas, Lawrence, Kansas 66047-3729; and Rocky MountainHerbarium, Department of Botany,University of Wyoming,Laramie, Wyoming82071-3165 Rayjacksonia R. L. Hartman& M. A. Lane, gen. nov. (Compositae: Astereae), is named to accommodate the "phyllo- cephalus complex," formerlyof Haplopappus Cass. sect. Blepharodon DC. The new combinationsare R. phyllocephalus (DC.) R. L. Hartman& M. A. Lane, R. annua (Rydb.) R. L. Hartman& M. A. Lane, and R. aurea (A. Gray) R. L. Hartman & M. A. Lane. This transfercompletes the reclassificationof the North American species of Haplopappus sensu Hall, leaving that genus exclusively South American.Rayjacksonia has a base chromosomenumber of x = 6. Furthermore,it shares abruptlyampliate disk corollas, deltatedisk style-branchappendages, and corolla epidermalcell type,among other features,with Grindelia, Isocoma, Olivaea, Prionopsis, Stephanodoria, and Xanthocephalum.Phylogenetic analyses of morphologicaland chloroplastDNA restrictionsite data, taken together,demonstrate that these genera are closely related but distinct. Key words: Astereae; Asteraceae; Compositae; Haplopappus; Rayjacksonia. During the past seven decades, taxonomic application lopappus sensu Hall (1928) are reclassifiedand are cur- -

Jessica's Aster
Jessica’s Aster (Aster jessicae) Population Monitoring: Third-year Results Idaho Conservation Data Center Idaho Department of Fish and Game PO Box 25 Juanita Lichthardt Boise, Idaho Karen Gray 83707 2005 Report prepared for the U.S. Fish and Wildlife Service ABSTRACT Jessica’s aster (Aster jessicae) is a tall, rhizomatous aster endemic to the Palouse region of southeastern Washington and adjacent Idaho. Its habitat has been severely reduced by conversion of this region to intensive agricultural uses. It is restricted almost entirely to private lands, and because of this, little was known of its distribution and abundance until a 1991 status survey was conducted. Five permanent monitoring plots were established in 2001, to track and compare populations occupying contrasting sites. Monitoring sites differ in amount of edge, degree of isolation from other populations, habitat extent, and amount of forest cover. This report summarizes three consecutive years of monitoring data. i TABLE OF CONTENTS ABSTRACT ......................................................................................................................................i TABLE OF CONTENTS ................................................................................................................ ii LIST OF TABLES ......................................................................................................................... iii LIST OF FIGURES....................................................................................................................... -

FLORATECH New Crop Report
FLORATECH New Crop Report presents: Machaeranthera gracilis Slender Goldenweed Taxonomy Machaeranthera gracilis (Nutt.) Shinners Translated: sword-like anthers; graceful. A.k.a.: Dieteria gracilis Haplopappus gracilis Haplopappus ravenii Sideranthus gracilis And newly named Xanthisma gracile WITHIN ASTERACEAE GEOGRAPHIC DISTRIBUTION Native to CA, NV, UT, AZ, CO, NM, TX, ME, NY according to USDA NRCS No reports of naturalizing Climactic Conditions5: Sandy or rocky places to 5000’ Habitats: creosote bush scrub, joshua tree woodland, east Mojave Desert Taxonomic Description7 Annual with erect stems, 3.25cm, leafy, branched at or above base, bristly throughout. Leaves: 1-3cm long, 3-7mm wide; lower oblanceolate, elliptic, or oblong in outline, 1-2 pinnately lobed; upper linear, reduced, lobes and teeth bristle-tipped. Inflorescence: heads radiate, solitary or in cymes; involucre 6-7mm, 7-12mm wide, hemispheric; phyllaries (bracts) in rows of 4-6, linear-lanceolate, bristle-tipped, hairy. Flowering time often from April-June. Ray flower: 16-18; ligules 7-12mm, yellow. Disk flower: 44-65; corollas 4.5-5.5mm, yellow. Taxonomic Description cont. Machaeranthera spp. tend to have several short rhizomes arising from the caudex (woody base of a perennial).1 Rhizomes may or may not function in vegetative reproduction.1 Biseasonal (germinates in winter, but flowers and sets seed in summer.2 Seed set greatly varied from season to season and shows significant responses to microhabitat differentiation.2 Use by indigenous people The Ramah Navaho used M. gracilis3: In a cold infusion used as lotion for pimples, boils and sores. In a cold, compound infusion of plant used as an eyewash. -

Asters of Yesteryear (Updated April 2018)
Asters of Yesteryear (Updated April 2018) About this Update: The document was originally posted in a shorter version, to accompany the brief article "Where Have all our Asters Gone?" in the Fall 2017 issue of Sego Lily. In that version it consisted simply of photos of a number of plants that had at some time been included in Aster but that no longer are, as per Flora of North America. In this version I have added names to the photos to indicate how they have changed since their original publication: Date and original name as published (Basionym) IF name used in Intermountain Flora (1994) UF name used in A Utah Flora (1983-2016) FNA name used in Flora of North America (2006) I have also added tables to show the renaming of two groups of species in the Astereae tribe as organized in Intermountain Flora. Color coding shows how splitting of the major genera largely follows fault lines already in place No color Renamed Bright Green Conserved Various Natural groupings $ Plant not in Utah It is noteworthy how few species retain the names used in 1994, but also how the renaming often follows patterns already observed. Asters of Yesteryear (Updated April 2018) Here are larger photos (16 inches wide or tall at normal screen resolution of 72 dpi) of the plants shown in Sego Lily of Fall 2017, arranged by date of original publication. None of them (except Aster amellus on this page) are now regarded as true asters – but they all were at one stage in their history. Now all are in different genera, most of them using names that were published over 100 years ago. -

Report on the Conservation Status of Haplopappus Radiatus, in Idaho
REPORT ON THE CONSERVATION STATUS OF HAPLOPAPPUS RADIATUS, IN IDAHO by Michael Mancuso and Robert K. Moseley Conservation Data Center Nongame and Endangered Wildlife Program June, 1993 Idaho Department of Fish and Game 600 South Walnut, P.O. Box 25 Boise, Idaho 83707 Jerry M. Conley, Director Status Survey Report prepared for Idaho Department of Parks and Recreation through Section 6 funding from U.S. Fish and Wildlife Service, Region 1 REPORT ON THE CONSERVATION STATUS OF i HAPLOPAPPUS RADIATUS, IN IDAHO Taxon Name: Haplopappus radiatus (Nutt.) Cronq. Common Name: Snake River goldenweed Family: Asteraceae (Compositae) States Where Taxon Occurs: U.S.A.; Idaho, Oregon Current Federal Status: Category 1 Candidate Recommended Federal Status: Threatened Authors of Report: Michael Mancuso and Robert K. Moseley Original Date of Report: June 18, 1993 Date of Most Recent Revision: N/A Individual to Whom Further Information and Comments Should be Sent: Robert K. Moseley Conservation Data Center Idaho Dept. Fish and Game P.O. Box 25 Boise, ID 83707 ii ABSTRACT Snake River goldenweed (Haplopappus radiatus) has been recognized as a possible conservation concern for at least twenty years. Until 1991, field inventories in Idaho were limited. In 1991, the Idaho Conservation Data Center completed a field investigation for Snake River goldenweed on the Payette National Forest. Through funding provided by the U.S. Fish and Wildlife Service, additional fieldwork was completed throughout the rest of the species' range in Idaho, during 1992. Survey work has also been done in Oregon. Besides field inventories, research biologists associated with the Conservation Biology Program, Oregon Department of Agriculture, have done cytological and pollination investigations, and presently are monitoring several populations in Oregon. -

Haplopappus Crispus and H. Zionis (Asteradeae): New Species from Utah
Great Basin Naturalist Volume 43 Number 2 Article 2 4-30-1983 Haplopappus crispus and H. zionis (Asteradeae): new species from Utah Loran C. Anderson Florida State University, Tallahassee, Florida Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Anderson, Loran C. (1983) "Haplopappus crispus and H. zionis (Asteradeae): new species from Utah," Great Basin Naturalist: Vol. 43 : No. 2 , Article 2. Available at: https://scholarsarchive.byu.edu/gbn/vol43/iss2/2 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. HAPLOPAPPUS CRISPUS AND H. ZIONIS (ASTERACEAE): NEW SPECIES FROM UTAH Loran C. Anderson' Abstract. — The new species, Haplopappus crispus and H. zionis of section Macronema, are formally described and illustrated. They are endemic to southern Utah. Also, H. bloomeri ssp. compactus is raised to species. Chromo- .some numbers of all three are n = 9. Aspects of anatomy are detailed. Comparisons are made to H. bloomeri and H. suffntticosus. Relationships are discussed, and a key to the species is given. The only comprehensive monograph of compactus: Ackerman 30797, m (FSU), An- Haplopappus is that of H. M. Hall (1928). In derson 6186, a and m (FSU), Clokey 8570, m recent years the generic integrity of Hap- (UTC); H. crispus: Anderson 5504, a and m lopappus has been questioned (see Anderson (FSU), Cottam 1526, m (BRY), Maguire 1980 for review). -

Redalyc.Biomass, Resin and Essential Oil Content and Their Variability In
Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas ISSN: 0717-7917 [email protected] Universidad de Santiago de Chile Chile GONZÁLEZ, Benita; VOGEL, Hermine; RAZMILIC, Iván; MARTÍN, José San; DOLL, Ursula Biomass, resin and essential oil content and their variability in natural populations of the Chilean crude drug "bailahuén" (Haplopappus spp.) Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, vol. 11, núm. 1, 2012, pp. 66-73 Universidad de Santiago de Chile Santiago, Chile Available in: http://www.redalyc.org/articulo.oa?id=85622229007 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative © 2012 Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 11 (1): 66 - 73 ISSN 0717 7917 www.blacpma.usach.cl Artículo Original | Original Article Biomass, resin and essential oil content and their variability in natural populations of the Chilean crude drug “bailahuén” (Haplopappus spp.) [Biomasa, contenido de resina y aceite esencial y su variabilidad en poblaciones naturales de las especies de droga cruda chilena “bailahuén” (Haplopappus spp.)] Benita GONZÁLEZ1, Hermine VOGEL1, Iván RAZMILIC2, José San MARTÍN3 & Ursula DOLL4 1 Facultad de Ciencias Agrarias, Universidad de Talca, Talca, Chile. 2 Instituto de Química de Recursos Naturales, Universidad de Talca, Talca, Chile. 3 Instituto de Biología Vegetal y Biotecnología, Universidad de Talca, Talca, Chile. 4 Facultad de Ciencias Forestales, Universidad de Talca, Talca, Chile. Contactos | Contacts: Benita GONZALEZ - E-mail address: [email protected] Abstract Bailahuén (Haplopappus rigidus, Haplopappus baylahuen, Haplopappus multifolius and Haplopappus taeda; Asteraceae) are medicinal shrubs native to the Andes Mountains of Chile widely used to treat hepatic ailments. -

Asteraceae)§ Karin M.Valant-Vetscheraa and Eckhard Wollenweberb,*
Chemodiversity of Exudate Flavonoids in Seven Tribes of Cichorioideae and Asteroideae (Asteraceae)§ Karin M.Valant-Vetscheraa and Eckhard Wollenweberb,* a Department of Plant Systematics and Evolution Ð Comparative and Ecological Phytochemistry, University of Vienna, Rennweg 14, A-1030 Wien, Austria b Institut für Botanik der TU Darmstadt, Schnittspahnstrasse 3, D-64287 Darmstadt, Germany. E-mail: [email protected] * Author for correspondence and reprint requests Z. Naturforsch. 62c, 155Ð163 (2007); received October 26/November 24, 2006 Members of several genera of Asteraceae, belonging to the tribes Mutisieae, Cardueae, Lactuceae (all subfamily Cichorioideae), and of Astereae, Senecioneae, Helenieae and Helian- theae (all subfamily Asteroideae) have been analyzed for chemodiversity of their exudate flavonoid profiles. The majority of structures found were flavones and flavonols, sometimes with 6- and/or 8-substitution, and with a varying degree of oxidation and methylation. Flava- nones were observed in exudates of some genera, and, in some cases, also flavonol- and flavone glycosides were detected. This was mostly the case when exudates were poor both in yield and chemical complexity. Structurally diverse profiles are found particularly within Astereae and Heliantheae. The tribes in the subfamily Cichorioideae exhibited less complex flavonoid profiles. Current results are compared to literature data, and botanical information is included on the studied taxa. Key words: Asteraceae, Exudates, Flavonoids Introduction comparison of accumulation trends in terms of The family of Asteraceae is distributed world- substitution patterns is more indicative for che- wide and comprises 17 tribes, of which Mutisieae, modiversity than single compounds. Cardueae, Lactuceae, Vernonieae, Liabeae, and Earlier, we have shown that some accumulation Arctoteae are grouped within subfamily Cichori- tendencies apparently exist in single tribes (Wol- oideae, whereas Inuleae, Plucheae, Gnaphalieae, lenweber and Valant-Vetschera, 1996). -

Snake River Goldenweed (Pyrrocoma Radiata)
Snake River goldenweed (Pyrrocoma radiata) ENDANGERED Flowers (left), habit (center), and habitat (right) of Snake River goldenweed. Photos by ODA staff (left and center) and Rebecca Currin (right). If downloading images from this website, please credit the photographer. Family Asteraceae Taxonomic notes Synonym: Haplopappus radiatus Plant description Snake River goldenweed is a perennial species with one to several stems 30-100 cm tall arising from a woody taproot. The plant is essentially glabrous throughout. Basal leaves are tufted, broadly elliptic, usually 15-50 cm long (including the petiole) and 5- 20 cm wide. The numerous cauline leaves are sharply toothed and reduced, becoming sessile above, the lower leaves obovate, the upper ovate with a clasping base. Flowering heads are 2.5-4 cm wide and number 1-12, usually in an open corymbiform arrangement, the involucres approximately 2.5 cm high, with ovate-oblong, pale- margined loose bracts. Ray and disk florets are yellow, the ray florets 0.6-1.2 cm long and numbering 17-50, the disk corollas approximately 1.5 cm long, disk florets numbering 80-100 or more. The achenes are elongate with 40-60 rigid, unequal brownish pappus bristles. Distinguishing characteristics Snake River goldenweed is most closely related to Pyrrocoma carthamoides, which also occurs in the Snake River canyon. Snake River goldenweed is distinguished from this more widespread congener by glabrous stems and much wider basal leaves (5-20 cm wide versus 0.5-4 cm in P. carthamoides). When to survey Surveys should be completed when Snake River goldenweed is flowering, from June through July. -

Karyotype Traits in Grindelia Squarrosa (Pursh) Dunal (Asteraceae), an Invasive Plant in Romania
Truta et. al.·Silvae Genetica (2012) 61-4/5, 179-186 Karyotype traits in Grindelia squarrosa (Pursh) Dunal (Asteraceae), an invasive plant in Romania By ELENA TRUTA1),*), GABRIELA VOCHITA1), ADRIAN OPREA2) and CULITA SIRBU3) (Received 3rd February 2012) Abstract Europe (Russia, the Ukraine, Republic of Moldova, Esto- The description of the karyotype features and idio- nia, Lithuania, Czech Republic, Belgium, Sweden, gram in Grindelia squarrosa (Pursh) Dunal (Aster- Latvia, Ireland) (SIRBU and OPREA, 2008). Although the aceae), an invasive plant in Romania, are reported here species is most common in the lower elevations of plains for the first time. The diploid chromosome number is and foothills, it was met, too, at 3000 m altitude in Col- 2n = 2x = 12, in agreement with the data published for orado and New Mexico. In Northern Utah, it occurs in the other species of the genus. The karyomorphological Tony Grove Canyon (2000 m) in the Cache National For- data show that the complements of the studied geno- est, also on disturbed ground throughout the valleys and types have small chromosomes (mean chromosome – in the adjacent Wasatch Mountains at elevations of at length is ± SE = 2.56 ± 0.10 µm, and mean length of X – least 2200 m (MCDONOUGH, 1975; WALSH, 1993). Report- haploid complements is ± SE = 15.33 ± 0.69 µm, with a X ed for the first time in the flora of Romania in 1998 range of variability comprised between 12.87–17.51 µm). The karyotypes are made up of six pairs of metacentric (SIRBU and OPREA, 1998), G. squarrosa can be considered and submetacentric chromosomes, with an identical an invasive alien plant in this country.