(Diptera, Cecidomyiidae) on Haplopappus Foliosus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

W. DENNIS CLARK, Phd CURRICULUM VITAE
W. DENNIS CLARK, PhD CURRICULUM VITAE Address 4701 E. Wintu Way Phoenix, AZ 85044 Phone 480-730-6688 (office); 602-908-8520 (cell) E-Mail [email protected] EDUCATION Ph.D. in Botany (Plant Chemistry), 1977, University of Texas B.A. in Biological Sciences, 1970, Sacramento State College PROFESSIONAL EXPERIENCE 1976-2006 Faculty, Dept. of Plant Biology, Arizona State University 2004-present Adjunct Professor, Southwest College of Naturopathic Medicine 1989-1990 Visiting Faculty, Department of Botany and Plant Sciences, University of California at Riverside 1983-1984 Guest Professor, Institut für Pharmazeutische Biologie, Universität Heidelberg 1976-1981 Assistant Professor of Botany, Arizona State University 1975-1976 Assistant Instructor and Welch Foundation Research Fellow, University of Texas 1971-1972 Assistant Herbarium Curator, Sacramento State College 1970-1971 Graduate Assistant, Sacramento State College 1969-1971 Research Assistant, Sacramento State College PROFESSIONAL SERVICE Scientific Advisor, Center for the Study of Carbon Dioxide and Global Change, Tempe, AZ Institutional Review Board, Southwest College of Naturopathic Medicine, Tempe, AZ Strategic Advisor, Nutri-Health Supplements, Cottonwood, AZ Scientific Advisor, Larrea Biosciences Corp, Houston, TX COURSES TAUGHT Plant Chemistry and Pharmacognosy General Botany/Plants & Society/Concepts in Plant Biology Survey of the Plant Kingdom/Comparative Plant Diversity Plant Secondary Metabolism Medical Botany Chemosystematics Laboratory Methods in Chemosystematics Perspectives in Plant Research (Molecular Systematics) Ethnopharmacology Integrative Medicine and Society GRADUATE STUDENTS SUPERVISED Charles H. Pickett, 1978 (MS: Pugnacious ants on Opuntia acanthocarpa) Bruce D. Parfitt, 1981 (MS: Systematics of the Opuntia curvospina complex) Gregory K. Brown, 1981 (PhD: Systematics of Haplopappus Section Gymnocoma) Michael J. Knox, 1983 (MS: -Phenethylamine Variation in Mammillaria) George A. -

Notes on Variation and Geography in Rayjacksonia Phyllocephala (Asteraceae: Astereae)
Nesom, G.L., D.J. Rosen, and S.K. Lawrence. 2013. Notes on variation and geography in Rayjacksonia phyllocephala (Asteraceae: Astereae). Phytoneuron 2013-53: 1–15. Published 12 August 2013. ISSN 2153 733X NOTES ON VARIATION AND GEOGRAPHY IN RAYJACKSONIA PHYLLOCEPHALA (ASTERACEAE: ASTEREAE) GUY L. NESOM 2925 Hartwood Drive Fort Worth, Texas 76109 [email protected] DAVID J. ROSEN Department of Biology Lee College Baytown, Texas 77522-0818 [email protected] SHIRON K. LAWRENCE Department of Biology Lee College Baytown, Texas 77522-0818 ABSTRACT Inflorescences of Rayjacksonia phyllocephala in the disjunct Florida population system are characterized by heads on peduncles with leaves mostly reduced to linear bracts; heads in inflorescences of the Mexico-Texas-Louisiana system are immediately subtended by relatively unreduced leaves. The difference is consistent and justifies recognition of the Florida system as R. phyllocephala var. megacephala (Nash) D.B. Ward. Scattered waifs between the two systems are identified here as one or the other variety, directly implying their area of origin. In the eastern range of var. phyllocephala , at least from Brazoria County, Texas, eastward about 300 miles to central Louisiana, leaf margins vary from entire to deeply toothed-spinulose. In contrast, margins are invariably toothed-spinulose in var. megacephala as well as in the rest of southeastern Texas (from Brazoria County southwest) into Tamaulipas, Mexico. In some of the populations with variable leaf margins, 70-95% of the individuals have entire to mostly entire margins. KEY WORDS : Rayjacksonia , morphological variation, leaf margins, disjunction, waifs Rayjacksonia phyllocephala (DC.) Hartman & Lane (Gulf Coast camphor-daisy) is an abundant and conspicuous species of the shore vegetation around the Gulf of Mexico. -

"Catálogo De Plantas Vasculares Del Cono Sur", Para La Flora De Chile
Boletín del Museo Nacional de Historia Natural, Chile, 62: 167-201 (2013) COMPLEMENTO Y CORRECCIONES AL “CATÁLOGO DE PLANTAS VASCULARES DEL CONO SUR”, PARA LA FLORA DE CHILE Mélica Muñoz-Schick1 y Vanezza Morales2 1 [email protected]; [email protected] RESUMEN Se presenta una lista de 523 nombres descritos para la fl ora de Chile que no fueron incluidos en las versiones impresas o digitales del “Catálogo de Plantas Vasculares del Cono Sur”. Del mismo modo se incorporan 47 nombres que presentan errores de escritura o en la autoridad. Para cada uno de ellos se muestra la publicación original y su validez (aceptado, sinónimo o no resuelto). PALABRAS CLAVE: Complemento al Catálogo Cono Sur, fl ora de Chile ABSTRACT A list of 523 additional names for the Chilean Flora is presented, which were omitted from the “Ca- tálogo de Plantas Vasculares del Cono Sur”. We also include 47 corrected names (spelling or author name errors). For each of the new names presented we include the original publication and their status (accepted, synonymous or not resolved). KEYWORDS: Supplement to the Cono Sur Checklist, Chilean Flora INTRODUCCIÓN En el transcurso del proyecto apoyado por la Mellon Foundation para el MNHN “Digitalización de Tipos de plantas vasculares del herbario SGO” [plants.jstor.org ], se trató de indicar la determinación actualizada para los especímenes de la colección; para esto nos basamos en el Catálogo de Plantas Vasculares del Cono Sur (Zuloaga et al. 2008) que comprende las plantas de la Argentina, Paraguay, Uruguay, sur de Brasil y Chile; muchas veces ello se complementó con las determinaciones anotadas en los mismos ejemplares, por los especialistas de cada género. -
1 C M 2 B H 3 B M 4 C M 5 B L 6 B M 7 B M 8
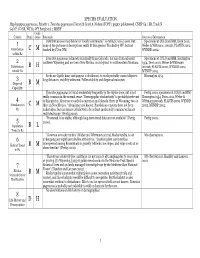
SPECIES EVALUATION Haplopappus pygmaeus, Priority 1. Tonestus pygmaeus (Torrey & Gray) A. Nelson (TOPY). pygmy goldenweed. CNHP G4 / SR, Track N G4 N?. CO SR, WY S1. WY Peripheral 2 MBNF Confi- Criteria Rank dence Rationale Sources of Information Distribution (see map below) is “nearly continuous,” so rating C was chosen, but Specimens at COLO and RM, Dorn 2001, 1 none of the pictures or descriptions really fit this species. Tracked by WY, but not Weber & Wittmann 2001ab, PLANTS 2002, Distribution C M tracked by CO or NM. WYNDD 2002. within R2 Tonestus pygmaeus is known principally from Colorado, but also from adjacent Specimens at COLO and RM, Harrington 2 southern Wyoming and northern New Mexico, and disjunct in southwestern Montana. 1954, Dorn 2001, Weber & Wittmann Distribution B H 2001ab, PLANTS 2002, WYNDD 2002, outside R2 MTNHP 2002. Seeds are lightly hairy and pappus is deciduous, so seeds probably cannot disperse Harrington 1954. 3 long distances; viability unknown. Pollen viability and dispersal unknown. Dispersal B M Capability Tonestus pygmaeus is found moderately frequently in the Alpine zone, but is not Fertig 2000, specimens at COLO and RM, really common in the normal sense. “Demographic stochasticity” is probably irrelevant Harrington 1954, Dorn 2001, Weber & 4 to this species. About 50 recorded occurrences in Colorado, three in Wyoming, two or Wittmann 2001ab, PLANTS 2002, WYNDD Abundance in C M three in New Mexico. “Abundance not known. Population censuses have not been 2002, MTNHP 2002. R2 undertaken, but the species is believed to be at least moderately common within its restricted range” (Fertig 2000). -

Reclassification of North American Haplopappus (Compositae: Astereae) Completed: Rayjacksonia Gen
AmericanJournal of Botany 83(3): 356-370. 1996. RECLASSIFICATION OF NORTH AMERICAN HAPLOPAPPUS (COMPOSITAE: ASTEREAE) COMPLETED: RAYJACKSONIA GEN. NOV.1 MEREDITH A. LANE2 AND RONALD L. HARTMAN R. L. McGregor Herbarium(University of Kansas NaturalHistory Museum Division of Botany) and Departmentof Botany,University of Kansas, Lawrence, Kansas 66047-3729; and Rocky MountainHerbarium, Department of Botany,University of Wyoming,Laramie, Wyoming82071-3165 Rayjacksonia R. L. Hartman& M. A. Lane, gen. nov. (Compositae: Astereae), is named to accommodate the "phyllo- cephalus complex," formerlyof Haplopappus Cass. sect. Blepharodon DC. The new combinationsare R. phyllocephalus (DC.) R. L. Hartman& M. A. Lane, R. annua (Rydb.) R. L. Hartman& M. A. Lane, and R. aurea (A. Gray) R. L. Hartman & M. A. Lane. This transfercompletes the reclassificationof the North American species of Haplopappus sensu Hall, leaving that genus exclusively South American.Rayjacksonia has a base chromosomenumber of x = 6. Furthermore,it shares abruptlyampliate disk corollas, deltatedisk style-branchappendages, and corolla epidermalcell type,among other features,with Grindelia, Isocoma, Olivaea, Prionopsis, Stephanodoria, and Xanthocephalum.Phylogenetic analyses of morphologicaland chloroplastDNA restrictionsite data, taken together,demonstrate that these genera are closely related but distinct. Key words: Astereae; Asteraceae; Compositae; Haplopappus; Rayjacksonia. During the past seven decades, taxonomic application lopappus sensu Hall (1928) are reclassifiedand are cur- -

Diptera: Cecidomyiidae) on Artemisia Species (Asteraceae
九州大学学術情報リポジトリ Kyushu University Institutional Repository Generic Position of Two Unidentified Japanese Gall Midges (Diptera: Cecidomyiidae) on Artemisia Species (Asteraceae) Nohara, Machiko Yukawa, Junichi http://hdl.handle.net/2324/2670 出版情報:ESAKIA. 43, pp.27-33, 2003-03-31. 九州大学大学院農学研究院昆虫学教室 バージョン: 権利関係: ESAKIA, (43): 27 - 33. March 31, 2003 Generic Position of Two Unidentified Japanese Gall Midges (Diptera: Cecidomyiidae) on Artemisia Species (Asteraeeae)* Machiko NOHARA Entomological Laboratory, Graduate School of Bioresource and Bioenvironmental Sciences, Kyushu University, Fukuoka, 81 2-858 1 Japan and Junichi YUKAWA Entomological Laboratory, Faculty of Agriculture, Kyushu University, Fukuoka, 812-8581 Japan Abstract. Among 19 Japanese gall midge species on Artemisia, 3 species that were knownonly from galls and larvae have been left unnamed. The morphological study proved that the 2 of them belonged to the genus Rhopalomyia of the tribe Rhopalomyiini. The larvae of both species are characterized by the reduction of terminal papillae from eight to six, the absence of sternal spatula, and reduction in the numberof lateral papillae. They were likely to be new species, but were not described because their adult specimens were not available. Unfortunately, remaining 1 species could not be studied due to the lack of specimens. As a result, the number of Japanese Rhopalomyia species on Artemisia totaled up to 14. This number is remarkably large for the species associated with a single plant genus. Key words: Rhopalomyia, gall midge, Rhopalomyiini, larval morphology, sternal spatula, identification. Introduction At least 19 species of gall midges (Diptera: Cecidomyiidae) that produce galls on the genus Artemisia (Asteraceae) have been known to occur in Japan (Yukawa & Masuda, 1996; Table 1). -

Differences in Arthropods Found in Flowers Versus Trapped in Plant
Arthropod-Plant Interactions (2014) 8:411–419 DOI 10.1007/s11829-014-9328-x ORIGINAL PAPER Differences in arthropods found in flowers versus trapped in plant resins on Haplopappus platylepis Phil. (Asteraceae): Can the plant discriminate between pollinators and herbivores? Cristian A. Villagra • Alvaro Astudillo Meza • Alejandro Urzu´a Received: 30 September 2013 / Accepted: 25 August 2014 / Published online: 26 September 2014 Ó Springer Science+Business Media Dordrecht 2014 Abstract Plants produce secondary metabolites related to insects we observed foraging on disk florets; Arthrobracus ecologically relevant processes. These compounds include (Coleoptera: Melyridae) and Linepithema (Hymenoptera: surface secretions such as latex, mucilage and resins that Formicidae) were the predominant insects ‘‘trapped in help plants face abiotic and biotic environmental threats resin’’, while Diadasia (Hymenoptera: Apidae) was the such as drought, nutrient deficiency, extreme temperatures most frequent ‘‘floral visitor’’. We propose that bracteal and UV radiation, as well as herbivory, pathogenic resin of H. platylepis may function as a selective trap for microorganisms and other natural enemies. We studied the non-mutualistic insects reaching reproductive structures of resinous coating found on involucral bracts of Haplopap- this plant and discuss other multiple possible roles for this pus platylepis Phil. (Asteraceae). This plant belongs to a secretion, including protocarnivory. speciose genus widely distributed in South America (Lane and Hartman in Am J Bot 83:356, 1996). H. platylepis is Keywords Constitutive resistance Á Terpenoids Á characterized by resinous fragrant leaves. In this species, Larcenist Á Diadasia chilensis Á Lioptilodes friasi resins cover the involucral bracts as well as young leaves and are also secreted on reproductive stalks in smaller amounts. -

Jessica's Aster
Jessica’s Aster (Aster jessicae) Population Monitoring: Third-year Results Idaho Conservation Data Center Idaho Department of Fish and Game PO Box 25 Juanita Lichthardt Boise, Idaho Karen Gray 83707 2005 Report prepared for the U.S. Fish and Wildlife Service ABSTRACT Jessica’s aster (Aster jessicae) is a tall, rhizomatous aster endemic to the Palouse region of southeastern Washington and adjacent Idaho. Its habitat has been severely reduced by conversion of this region to intensive agricultural uses. It is restricted almost entirely to private lands, and because of this, little was known of its distribution and abundance until a 1991 status survey was conducted. Five permanent monitoring plots were established in 2001, to track and compare populations occupying contrasting sites. Monitoring sites differ in amount of edge, degree of isolation from other populations, habitat extent, and amount of forest cover. This report summarizes three consecutive years of monitoring data. i TABLE OF CONTENTS ABSTRACT ......................................................................................................................................i TABLE OF CONTENTS ................................................................................................................ ii LIST OF TABLES ......................................................................................................................... iii LIST OF FIGURES....................................................................................................................... -

Evolutionary Diversification of the Gall Midge Genus Asteromyia
Molecular Phylogenetics and Evolution 54 (2010) 194–210 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Evolutionary diversification of the gall midge genus Asteromyia (Cecidomyiidae) in a multitrophic ecological context John O. Stireman III a,*, Hilary Devlin a, Timothy G. Carr b, Patrick Abbot c a Department of Biological Sciences, Wright State University, 3640 Colonel Glenn Hwy., Dayton, OH 45435, USA b Department of Ecology and Evolutionary Biology, Cornell University, E145 Corson Hall, Ithaca, NY 14853, USA c Department of Biological Sciences, Vanderbilt University, Box 351634 Station B, Nashville, TN 37235, USA article info abstract Article history: Gall-forming insects provide ideal systems to analyze the evolution of host–parasite interactions and Received 3 April 2009 understand the ecological interactions that contribute to evolutionary diversification. Flies in the family Revised 17 August 2009 Cecidomyiidae represent the largest radiation of gall-forming insects and are characterized by complex Accepted 9 September 2009 trophic interactions with plants, fungal symbionts, and predators. We analyzed the phylogenetic history Available online 16 September 2009 and evolutionary associations of the North American cecidomyiid genus Asteromyia, which is engaged in a complex and perhaps co-evolving community of interactions with host-plants, fungi, and parasitoids. Keywords: Mitochondrial gene trees generally support current classifications, but reveal extensive cryptic diversity Adaptive diversification within the eight named species. Asteromyia likely radiated after their associated host-plants in the Aste- Fungal mutualism Insect-plant coevolution reae, but species groups exhibit strong associations with specific lineages of Astereae. Evolutionary asso- Cryptic species ciations with fungal mutualists are dynamic, however, and suggest rapid and perhaps coordinated Parasitoid changes across trophic levels. -

Articles-38747 Archivo 01.Pdf
MINISTERIO DE EDUCACIÓN PUBLICA Ministro de Educación Pública Carolina Schmidt Zaldívar Subsecretario de Educación Fernando Rojas Ochagavía Dirección de Bibliotecas, Magdalena Krebs Kaulen Archivo y Museos Diagramación Herman Núñez Impreso por BOLETÍN DEL MUSEO NACIONAL DE HISTORIA NATURAL CHILE Director Claudio Gómez Papic Editor Herman Núñez Comité Editor Pedro Báez R. Mario Elgueta D. Gloria Rojas V. David Rubilar R. Rubén Stehberg L. (c) Dirección de Bibliotecas, Archivos y Museos Inscripción N° XXXXXXX Edición de 100 ejemplares Museo Nacional de Historia Natural Casilla 787 Santiago de Chile www.mnhn.cl Se ofrece y acepta canje Exchange with similar publications is desired Échange souhaité Wir bitten um Austach mit aehnlichen Fachzeitschriften Si desidera il cambio con publicazioni congeneri Deseja-se permuta con as publicações congéneres Este volumen se encuentra disponible en soporte electrónico como disco compacto y en línea en Contribución del Museo Nacional de Historia Natural al Programa del Conocimiento y Preservación de la Diversidad Biológica Las opiniones vertidas en cada uno de los artículos publicados son de excluisiva responsabilidad del autor respectivo BOLETÍN DEL MUSEO NACIONAL DE HISTORIA NATURAL CHILE 2013 62 SUMARIO CLAUDIO GÓMEZ P. Editorial ............................................................................................................................................................................6 ANDRÉS O. TAUCARE-RÍOS y WALTER SIELFELD Arañas (Arachnida: Araneae) del Extremo Norte de Chile ...............................................................................................7 -

Diptera: Cecidomyiidae), Com Ênfase No Posicionamento Das Espécies Da Região Neotropical
ANTONIO MARCELINO DO CARMO NETO Sistemática das espécies da supertribo Stomatosematidi (Diptera: Cecidomyiidae), com ênfase no posicionamento das espécies da Região Neotropical São Paulo, 2017 ANTONIO MARCELINO DO CARMO NETO Sistemática das espécies da supertribo Stomatosematidi (Diptera: Cecidomyiidae), com ênfase no posicionamento das espécies da Região Neotropical Dissertação apresentada ao Programa de Pós- Graduação em Sistemática, Taxonomia Animal e Biodiversidade do Museu de Zoologia da Universidade de São Paulo para obtenção do Título de Mestre. Orientador: Prof. Dr. Carlos José Einicker Lamas São Paulo, 2017 i AUTORIZAÇÃO Não autorizo a reprodução e divulgação total ou parcial deste trabalho, por qualquer meio convencional ou eletrônico. I do not authorize the reproduction and dissemination of this work in part or entirely by any means eletronic or conventional. ii Carmo-Neto, Antonio Marcelino Sistemática das espécies da supertribo Stomatosematidi (Diptera: Cecidomyiidae), com ênfase no posicionamento das espécies da Região Neotropical / Antonio Marcelino do Carmo Neto; orientador: Carlos José Einicker Lamas – São Paulo, SP: 2017 106 p. Dissertação (Mestrado) – Programa de Pós-Graduação em Sistemática, Taxonomia Animal e Biodiversidade, Universidade de São Paulo, 2017. 1. Entomologia. I. Lamas, Carlos Jósé Einicker, orient. II. Título. iii Folha de Aprovação Banca Examinadora Prof. Dr. _____________Instituição: ______________ Julgamento: ___________ Assinatura: ______________ Prof. Dr. _____________Instituição: ______________ -

Taxonomy of Janetiella Thymi (Kieffer) (Diptera: Cecidomyiidae) and of the Species Formerly in Janetiella That Feed on Vitis (Vitaceae)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USDA Systematic Entomology Laboratory Entomology Collections, Miscellaneous 2009 Taxonomy of Janetiella thymi (Kieffer) (Diptera: Cecidomyiidae) and of the Species Formerly in Janetiella That Feed on Vitis (Vitaceae) Raymond Gagne Systematic Entomology Laboratory, PSI, Agricultural Research Service, USDA, c/o U. S. National Museum NHB 168, P.O. Box 37012, Washington, DC 20013-7012, USA, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/systentomologyusda Part of the Entomology Commons Gagne, Raymond, "Taxonomy of Janetiella thymi (Kieffer) (Diptera: Cecidomyiidae) and of the Species Formerly in Janetiella That Feed on Vitis (Vitaceae)" (2009). USDA Systematic Entomology Laboratory. 21. https://digitalcommons.unl.edu/systentomologyusda/21 This Article is brought to you for free and open access by the Entomology Collections, Miscellaneous at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USDA Systematic Entomology Laboratory by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. PROC. ENTOMOL. SOC. WASH. 111(2), 2009, pp. 399–409 TAXONOMY OF JANETIELLA THYMI (KIEFFER) (DIPTERA: CECIDOMYIIDAE) AND OF THE SPECIES FORMERLY IN JANETIELLA THAT FEED ON VITIS (VITACEAE) RAYMOND J. GAGNE´ Systematic Entomology Laboratory, PSI, Agricultural Research Service, U.S. Department of Agriculture, c/o Smithsonian Institution MRC-168, P.O. Box 37012, Washington, DC 20013-7012, U.S.A. (e-mail: [email protected]) Abstract.—The poorly known European species Janetiella thymi (Kieffer), type species of Janetiella Kieffer (Diptera: Cecidomyiidae), is redescribed. Gall makers on grape that were formerly placed in Janetiella are shown to be distinct from that genus and transferred to Vitisiella Fedotova & Kovalev, a genus recently erected for a species on grape in Siberia.