Pre - PA Allowance Age 12 Years of Age Or Older – Ultracet (Tramadol and Acetaminophen) and Codeine/APAP Products
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Opioid Powders Page: 1 of 9
Federal Employee Program® 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.64 Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Analgesics and Anesthetics Original Policy Date: October 20, 2017 Subject: Opioid Powders Page: 1 of 9 Last Review Date: March 13, 2020 Opioid Powders Description Buprenorphine Powder, Butorphanol Powder, Codeine Powder, Hydrocodone Powder, Hydromorphone Powder, Levorphanol Powder, Meperidine Powder, Methadone Powder, Morphine Powder, Oxycodone Powder, Oxymorphone Powder Background Pharmacy compounding is an ancient practice in which pharmacists combine, mix or alter ingredients to create unique medications that meet specific needs of individual patients. Some examples of the need for compounding products would be: the dosage formulation must be changed to allow a person with dysphagia (trouble swallowing) to have a liquid formulation of a commercially available tablet only product, or to obtain the exact strength needed of the active ingredient, to avoid ingredients that a particular patient has an allergy to, or simply to add flavoring to medication to make it more palatable. Buprenorphine, butorphanol, codeine, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxycodone, and oxymorphone powders are opioid drugs that are used for pain control. The intent of the criteria is to provide coverage consistent with product labeling, FDA guidance, standards of medical practice, evidence-based drug information, and/or published guidelines. Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, and because of the greater risks of overdose and death (1-15). Regulatory Status 5.70.64 Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Analgesics and Anesthetics Original Policy Date: October 20, 2017 Subject: Opioid Powders Page: 2 of 9 FDA-approved indications: 1. -

1 Impact of Opioid Agonists on Mental Health in Substitution
Impact of opioid agonists on mental health in substitution treatment for opioid use disorder: A systematic review and Bayesian network meta-analysis of randomized clinical trials Supplementary Table 1_ Specific search strategy for each database The following general combination of search terms, Boolean operators, and search fields were used where “*” means that any extension of that word would be considered: Title field [opium OR opiate* OR opioid OR heroin OR medication assisted OR substitution treatment OR maintenance treatment OR methadone OR levomethadone OR buprenorphine OR suboxone OR (morphine AND slow) OR diamorphine OR diacetylmorphine OR dihydrocodeine OR hydromorphone OR opium tincture OR tincture of opium OR methadol OR methadyl OR levomethadyl] AND Title/Abstract field [trial* OR random* OR placebo] AND All fields [depress* OR anxiety OR mental] Wherever this exact combination was not possible, a more inclusive version of the search strategy was considered. Database Search Strategy Ovid for EBM Reviews - Cochrane Central Register of (opium or opiate$ or opioid or heroin or medication Controlled Trials August 2018; Embase 1974 to assisted or substitution treatment or maintenance September 07, 2018; MEDLINE(R) and Epub Ahead treatment or methadone or levomethadone or of Print, In-Process & Other Non-Indexed Citations buprenorphine or suboxone or (morphine and slow) or and Daily 1946 to September 07, 2018 diamorphine or diacetylmorphine or dihydrocodeine or hydromorphone or opium tincture or tincture of opium or methadol or methadyl -

The Inhibition of Enkephalin Catabolism by Dual Enkephalinase Inhibitor: a Novel Possible Therapeutic Approach for Opioid Use Disorders
Alvarez-Perez Beltran (Orcid ID: 0000-0001-8033-3136) Maldonado Rafael (Orcid ID: 0000-0002-4359-8773) THE INHIBITION OF ENKEPHALIN CATABOLISM BY DUAL ENKEPHALINASE INHIBITOR: A NOVEL POSSIBLE THERAPEUTIC APPROACH FOR OPIOID USE DISORDERS ALVAREZ-PEREZ Beltran1*, PORAS Hervé 2*, MALDONADO Rafael1 1 Laboratory of Neuropharmacology, Department of Experimental and Health Sciences, Universitat Pompeu Fabra, Barcelona Biomedical Research Park, c/Dr Aiguader 88, 08003 Barcelona, Spain, 2 Pharmaleads, Paris BioPark, 11 Rue Watt, 75013 Paris, France *Both authors participated equally to the manuscript Correspondence: Rafael Maldonado, Laboratori de Neurofarmacologia, Universitat Pompeu Fabra, Parc de Recerca Biomèdica de Barcelona (PRBB), c/Dr. Aiguader, 88, 08003 Barcelona, Spain. E-mail: [email protected] ABSTRACT Despite the increasing impact of opioid use disorders on society, there is a disturbing lack of effective medications for their clinical management. An interesting innovative strategy to treat these disorders consists in the protection of endogenous opioid peptides to activate opioid receptors, avoiding the classical opioid-like side effects. Dual Enkephalinase Inhibitors (DENKIs) physiologically activate the endogenous opioid system by inhibiting the enzymes responsible for the breakdown of enkephalins, protecting endogenous enkephalins, increasing their half-lives and physiological actions. The activation of opioid receptors by the increased enkephalin levels, and their well-demonstrated safety, suggest that DENKIs could represent a novel analgesic therapy and a possible effective treatment for acute opioid withdrawal, as well as a promising alternative to opioid substitution therapy minimizing side effects. This new pharmacological class of compounds could bring effective and safe medications avoiding the This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. -

List of Narcotic Substances Circulation of Which Is Restricted in Uzbekistan
List of narcotic substances circulation of which is restricted in Uzbekistan 1. 2C-B(4-bromo-2,5-dimethoxyphenethylamine) 2. 3-methylfentanyl 3. 3-methylthiofentanyl 4. 3-Monoacetylmorphine 5. 4-methylaminorex 6. 6- Monoacetylmorphine 7. Acetorphine 8. Acetyl Dihydrocodeine 9. Acetyl-alfametilfentanil 10. Acetylated opium 11. Acetylcodeine 12. Acetylmethadol 13. Alfametadol 14. Alfatsetilmetadol 15. all fungi that contain Psilocine and Psilocybine 16. Allylprodine 17. Alpha Methylfentanyl 18. Alpha Metiltiofentanil 19. Alphaprodine 20. Anileridin 21. Benzethidine 22. Benzylmorphine 23. Betacetylmethadol 24. Betahydroxyfentanyl 25. Betameprodine 26. Betamethadol 27. Betaprodine 28. Bezitramide 29. Cannabis oil (hashish oil) 30. Cannabis, marihuana 31. Cathine ((+)-norpseudoephedrine) 32. Cathinone (l-alpha-aminopropiofenon) 33. Clonitazene 34. Cocaine 35. Codoxime 36. d- Methadone 37. DB [L-(3,4 - methylenedioxyphenyl) -2 butanamine] 38. Desmethylprodine; MPPP (1-methyl-4-phenyl-4-propionoxypiperidine) 39. Desomorphine 40. DET (N,N-diethyltryptamine) 41. Dexamphetamine 42. Diampromide 43. Diethyl phosphate 44. Diethylthiambutene 45. Dihydromorphine 46. Dimenoxadol 47. Dimepheptanol 48. Dimethylthiambutene 49. Dioxaphetyl butyrate 50. Diphenoxine 51. Dipipanone 52. DMA (2,5-dimethoxyamphetamine) 53. DMGP (dimetilgeptilpiran) 54. DMT (dimethyltryptamine) 55. DOB (d, L-2,5-dimethoxy-4-bromo-amphetamine) 56. DOC (d, L-2,5-dimethoxy-4-chloro-amphetamine) 57. DOET (2,5-dimethoxy-4-ethylamphetamine) 58. Drotebanol 59. Ecgonine 60. Ephedrone 61. Ethylmethylthiambutene 62. Eticyclidine 63. Etonitazene 64. Etorphine 65. Etoxeridine 66. Etryptamine 67. Furethidine 68. Hashish (Anasha, cannabis resin) 69. Heroin (Diacetylmorphine) 70. Hydrocodone 71. Hydrocodone phosphate 72. Hydromorphinol 73. Hydromorphone 74. Isomethadone 75. Ketobemidone 76. Khat 77. L- Methadone 78. Levomethorphan 79. Levomoramide 80. Levophenacylmorphan 81. Levorphanol 82. Lysergic acid and its preparations, that include d-Lysergide (LSD, LSD-25) 83. -

Effects of Medication-Assisted Treatment (MAT) on Functional Outcomes Among Patients with Opioid Use Disorder (OUD)
NATIONAL DEFENSE RESEARCH INSTITUTE Effects of Medication- Assisted Treatment (MAT) for Opioid Use Disorder on Functional Outcomes A Systematic Review Margaret A. Maglione, Laura Raaen, Christine Chen, Gulrez Shah Azhar, Nima Shahidinia, Mimi Shen, Ervant J. Maksabedian Hernandez, Roberta M. Shanman, Susanne Hempel Prepared for the Office of the Secretary of Defense Approved for public release; distribution unlimited For more information on this publication, visit www.rand.org/t/RR2108 Published by the RAND Corporation, Santa Monica, Calif. © Copyright 2018 RAND Corporation R® is a registered trademark. Limited Print and Electronic Distribution Rights This document and trademark(s) contained herein are protected by law. This representation of RAND intellectual property is provided for noncommercial use only. Unauthorized posting of this publication online is prohibited. Permission is given to duplicate this document for personal use only, as long as it is unaltered and complete. Permission is required from RAND to reproduce, or reuse in another form, any of its research documents for commercial use. For information on reprint and linking permissions, please visit www.rand.org/pubs/permissions. The RAND Corporation is a research organization that develops solutions to public policy challenges to help make communities throughout the world safer and more secure, healthier and more prosperous. RAND is nonprofit, nonpartisan, and committed to the public interest. RAND’s publications do not necessarily reflect the opinions of its research clients and sponsors. Support RAND Make a tax-deductible charitable contribution at www.rand.org/giving/contribute www.rand.org Preface Over the past two decades, the U.S. Department of Defense (DoD) has invested unparalleled resources into developing effective treatments for military-related psychological health conditions. -

Opioid-Containing Cough and Cold Products - Assessing the Potential Risk of Opioid Use Disorder and Related Harms in Children and Adolescents
Summary Safety Review –Opioid-containing cough and cold products - Assessing the potential risk of opioid use disorder and related harms in children and adolescents Product: Cough and cold products containing opioids (including codeine, hydrocodone or normethadone) Potential Safety Issue: Opioid use disorders and related harms in children and adolescents Key Messages • The growing number of overdoses and harms caused by opioids is a major public health concern in Canada. Cough and cold products containing opioids (including codeine, hydrocodone or normethadone) are authorized for sale in Canada to treat cough and cold symptoms in adults and children. • Health Canada reviewed the risk of opioid use disorder and related harms from these products after the United States Food and Drug Administration (US FDA) advised against using these products in children and adolescents in 2018. Health Canada’s safety review found limited evidence to link opioid-containing cough and cold products with opioid use disorders and related harms in children and adolescents. • These products are linked to other known harms (i.e., breathing problems), and there is limited evidence to support the effectiveness of these products in children and adolescents. There are other products available in Canada to help relieve the symptoms of cough and cold in children. • Therefore, Health Canada, as a precautionary measure, is advising Canadians against the use of these products among children and adolescents under 18 years of age. Health Canada will notify the manufacturers to update the product safety information of opioid- containing cough and cold products to limit the recommended age of use (indication) to adults only, 18 years of age and older. -

List of Narcotic Drugs Under International Control
International Narcotics Control Board Yellow List Annex to Forms A, B and C 59th edition, July 2020 LIST OF NARCOTIC DRUGS UNDER INTERNATIONAL CONTROL Prepared by the INTERNATIONAL NARCOTICS CONTROL BOARD* Vienna International Centre P.O. Box 500 A-1400 Vienna, Austria Internet address: http://www.incb.org/ in accordance with the Single Convention on Narcotic Drugs, 1961** Protocol of 25 March 1972 amending the Single Convention on Narcotic Drugs, 1961 * On 2 March 1968, this organ took over the functions of the Permanent Central Narcotics Board and the Drug Supervisory Body, r etaining the same secretariat and offices. ** Subsequently referred to as “1961 Convention”. V.20-03697 (E) *2003697* Purpose The Yellow List contains the current list of narcotic drugs under international control and additional relevant information. It has been prepared by the International Narcotics Control Board to assist Governments in completing the annual statistical reports on narcotic drugs (Form C), the quarterly statistics of imports and exports of narcotic drugs (Form A) and the estimates of annual requirements for narcotic drugs (Form B) as well as related questionnaires. The Yellow List is divided into four parts: Part 1 provides a list of narcotic drugs under international control in the form of tables and is subdivided into three sections: (1) the first section includes the narcotic drugs listed in Schedule I of the 1961 Convention as well as intermediate opiate raw materials; (2) the second section includes the narcotic drugs listed in Schedule II of the 1961 Convention; and (3) the third section includes the narcotic drugs listed in Schedule IV of the 1961 Convention. -

Narcotic Drugs Stupefiants Estupefacientes
E/INCB/1993/21Supp.6 INTERNATIONAL NARCOTICS CONTROL BOARD - VIENNA SUPPLEMENT No. 6 TO NARCOTIC DRUGS ESTIMATED WORLD REQUIREMENTS FOR 1994 STATISTICS FOR 1992 ESTIMATES UPDATED AS OF 30 JUNE 1994 ORGANE INTERNATIONAL DE CONTROLE DES STUPEFIANTS - VIENNE SUPPLEMENT N° 6 A r STUPEFIANTS EVALUATIONS DES BESOINS DU MONDE POUR 1994 STATISTIQUES POUR 1992 EVALUATIONS A JOUR AU 30 JUlN 1994 JUNTA INTERNACIONAL DE FISCALlZACION DE ESTUPEFACIENTES - VIENA SUPLEMENTO N.o 6 A ESTUPEFACIENTES PREVISIONES DE LAS NECESIDADES MUNDIALES PARA 1994 ESTADfsTICAS PARA 1992 PREVISIONES ACTUALlZADAS AL 30 DE JUNIO DE 1994 ~If..~~ ~ ~-tR UNITED NATIONS - NATIONS UNIES - NACIONES UNIDAS 1994 The updating of Table A is carried out by means of 12 monthly supplements. In order to facilitate the task of the exporting countries, the 12 supplements now report all the totals of the estimates and not only the amended data. In this way, each supplement cancels and replaces the published table in its entirety. In order to accelerate the transmission of the supplements to the competent national authorities, the 12 supplements will appear in English. Reading of these 12 supplements in French and Spanish may be facilitated by consulting the indexes of countries and territories and of narcotic drugs appearing in the annual publication. La mise El jour du tableau A s'effectue au moyen de douze supplements mensuels. Afin de faciliter la tache des pays exportateurs, les douze supplements contiennent tous les totaux des evaluations et non pas seulement les chiffres qui ont ete modifies. De celte maniere, chaque supplement annule et remplace entierement le tableau publie. -

Modulation of Opioid Transport at the Blood-Brain Barrier by Altered ATP-Binding Cassette (ABC) Transporter Expression and Activity
pharmaceutics Review Modulation of Opioid Transport at the Blood-Brain Barrier by Altered ATP-Binding Cassette (ABC) Transporter Expression and Activity Junzhi Yang 1, Bianca G. Reilly 2, Thomas P. Davis 1,2 and Patrick T. Ronaldson 1,2,* 1 Department of Pharmacology and Toxicology, College of Pharmacy, University of Arizona, 1295 N. Martin St., P.O. Box 210207, Tucson, AZ 85721, USA; [email protected] (J.Y.); [email protected] (T.P.D.) 2 Department of Pharmacology, College of Medicine, University of Arizona, 1501 N. Campbell Ave, P.O. Box 245050, Tucson, AZ 85724-5050, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-520-626-2173 Received: 19 September 2018; Accepted: 16 October 2018; Published: 18 October 2018 Abstract: Opioids are highly effective analgesics that have a serious potential for adverse drug reactions and for development of addiction and tolerance. Since the use of opioids has escalated in recent years, it is increasingly important to understand biological mechanisms that can increase the probability of opioid-associated adverse events occurring in patient populations. This is emphasized by the current opioid epidemic in the United States where opioid analgesics are frequently abused and misused. It has been established that the effectiveness of opioids is maximized when these drugs readily access opioid receptors in the central nervous system (CNS). Indeed, opioid delivery to the brain is significantly influenced by the blood-brain barrier (BBB). In particular, ATP-binding cassette (ABC) transporters that are endogenously expressed at the BBB are critical determinants of CNS opioid penetration. In this review, we will discuss current knowledge on the transport of opioid analgesic drugs by ABC transporters at the BBB. -

Hydromorphone Hydrochloride) CS-II WARNING: DILAUDID ORAL LIQUID and DILAUDID TABLETS CONTAIN HYDROMORPHONE, WHICH IS a POTENT SCHEDULE II CONTROLLED OPIOID AGONIST
DILAUDID® ORAL LIQUID and DILAUDID® TABLETS (hydromorphone hydrochloride) CS-II WARNING: DILAUDID ORAL LIQUID AND DILAUDID TABLETS CONTAIN HYDROMORPHONE, WHICH IS A POTENT SCHEDULE II CONTROLLED OPIOID AGONIST. SCHEDULE II OPIOID AGONISTS, INCLUDING MORPHINE, OXYMORPHONE, OXYCODONE, FENTANYL, AND METHADONE, HAVE THE HIGHEST POTENTIAL FOR ABUSE AND RISK OF PRODUCING RESPIRATORY DEPRESSION. ALCOHOL, OTHER OPIOIDS AND CENTRAL NERVOUS SYSTEM DEPRESSANTS (SEDATIVE-HYPNOTICS) POTENTIATE THE RESPIRATORY DEPRESSANT EFFECTS OF HYDROMORPHONE, INCREASING THE RISK OF RESPIRATORY DEPRESSION THAT MIGHT RESULT IN DEATH. DESCRIPTION Proprietary name: DILAUDID ORAL LIQUID Established name: hydromorphone hydrochloride Route of administration: ORAL (C38288) Active ingredients (moiety): hydromorphone hydrochloride (hydromorphone) # Strength Form Inactive ingredients 1 5 MILLIGRAM LIQUID purified water, methylparaben, propylparaben, sucrose, glycerin, sodium (C42953) metabisulfite Proprietary name: DILAUDID TABLETS Established name: hydromorphone hydrochloride Route of administration: ORAL (C38288) Active ingredients (moiety): hydromorphone hydrochloride (hydromorphone) # Strength Form Inactive ingredients 1 2 MILLIGRAM TABLET D&C red #30 Lake dye, D&C yellow #10 Lake dye, lactose, magnesium (C42998) stearate, sodium metabisulfite 2 4 MILLIGRAM TABLET D&C yellow #10 Lake dye, lactose, magnesium stearate, sodium metabisulfite (C42998) 3 8 MILLIGRAM TABLET lactose anhydrous, magnesium stearate, sodium metabisulfite (C42998) DILAUDID (hydromorphone hydrochloride), a hydrogenated ketone of morphine, is an opioid analgesic. The chemical name of DILAUDID (hydromorphone hydrochloride) is 4,5α-epoxy-3- hydroxy-17-methylmorphinan-6-one hydrochloride. The structural formula is: M.W. 321.8 Each 5 mL (1 teaspoon) of DILAUDID ORAL LIQUID contains 5 mg of hydromorphone hydrochloride. In addition, other ingredients include purified water, methylparaben, propylparaben, sucrose, and glycerin. DILAUDID ORAL LIQUID may contain traces of sodium metabisulfite. -
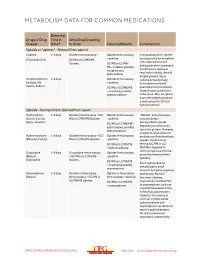
Metabolism Data for Common Medications
METABOLISM DATA FOR COMMON MEDICATIONS Detection Drugs or Drug Time in Urine Drug Screening Classes Urine* to Order Expected Results Consideration Opioids or “opiates” – Natural (from opium) Codeine 1–3 days Opiates Immunoassay + Opiates Immunoassay Immunoassays for “opiates” are responsive for morphine (Tylenol #2/3/4) GC/MS or LC/MS/MS – positive and codeine but do not Opiates GC/MS or LC/MS/ distinguish which is present. MS – codeine, possibly Confirmatory testing is morphine and required to reliably identify hydrocodone drug(s) present. Since Morphine (Avinza, 1–3 days Opiates Immunoassay codeine is metabolized Embeda, MS – positive to morphine and small Contin, Kadian) GC/MS or LC/MS/MS quantities to hydrocodone, – morphine, possibly these drugs may be found hydromorphone in the urine. Also, morphine may metabolize to produce a small amount (<10%) of hydromorphone. Opioids – Semisynthetic (derived from opium Hydrocodone 1–3 days Opiates Immunoassay + GC/ Opiates Immunoassay “Opiates” immunoassays (Lorcet, Lortab, MS or LC/MS/MS Opiates – positive may also detect Norco, Vicodin) semisynthetic opioids GC/MS or LC/MS/MS – depending on their cross- hydrocodone, possibly reactivity pattern. However, hydromorphone a negative result does not Hydromorphone 1–3 days Opiates Immunoassay + GC/ Opiates Immunoassay exclude use of semisynthetic (Dilaudid, Exalgo) MS or LC/MS/MS Opiates –positive opioids. Confirmatory GC/MS or LC/MS/MS testing (GC/MS or LC/ – hydromorphone MS/MS) is required to verify compliance with the Oxycodone 1–3 days Oxycodone Immunoassay Opiates Immunoassay prescribed semisynthetic (Roxicet, + GC/MS or LC/MS/MS –positive opioid(s). OxyContin) Opiates GC/MS or LC/MS/MS Since hydrocodone is – oxycodone possibly metabolized in small oxymorphone amounts to hydromorphone, Oxymorphone 1–3 days Opiates or Oxycodone Opiates or Oxycodone both may be found in (Opana) Immunoassay + GC/MS or Immunoassay – positive the urine. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0