The Effect of Preoperative Acetaminophen/Hydrocodone on the Efficacy of the Inferior Alveolar Nerve Block in Patients with Symptomatic Irreversible Pulpitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Opioids): SOR/2018-77 Canada Gazette, Part II, Volume 152, Number 9
Canada Gazette, Part 2, Volume 152, Number 9: Regulations Amendin... http://gazette.gc.ca/rp-pr/p2/2018/2018-05-02/html/sor-dors77-eng.html Home (http://www.canada.ca/en/index.html) How government works (http://www.canada.ca/en/government/system/index.html) Treaties, laws and regulations (https://www.canada.ca/en/government/system/laws.html) Canada Gazette (/accueil-home-eng.html) Publications (/rp-pr/publications-eng.html) Part II: Vol. 152 (2018) (/rp-pr/p2/2018/index-eng.html) May 2, 2018 (/rp-pr/p2/2018/2018-05-02/html/index-eng.html) Regulations Amending the Food and Drug Regulations (Opioids): SOR/2018-77 Canada Gazette, Part II, Volume 152, Number 9 Registration April 23, 2018 FOOD AND DRUGS ACT P.C. 2018-429 April 20, 2018 Her Excellency the Governor General in Council, on the recommendation of the Minister of Health, pursuant to section 30a of the Food and Drugs Act b, makes the annexed Regulations Amending the Food and Drug Regulations (Opioids). Regulations Amending the Food and Drug Regulations (Opioids) Amendments 1 Subsections C.01.005(2) and (3) of the Food and Drug Regulations 1 are replaced by the following: (2) Subsection (1) does not apply to (a) a drug in dosage form that is compounded by a pharmacist under a prescription or by a practitioner; or (b) a drug in dosage form that is sold under a prescription if the following information appears on the drug’s label: (i) the drug’s proper name, common name or brand name, (ii) the drug’s potency, and (iii) the name of the drug’s manufacturer. -

Oklahoma Drug Threat Assessment
2018 Oklahoma Drug Threat Assessment Oklahoma Bureau of Narcotics and Dangerous Drugs i John Scully, Director MARY FALLIN JOHN SCULLY Governor Director September 12, 2018 The Oklahoma Bureau of Narcotics has served the citizens of Oklahoma in the quest for a drug- free state since 1975. Our agency remains committed to working with lawmakers, law enforcement, public health officials, and the citizens of Oklahoma to develop comprehensive strategies to address drug abuse in communities across the state. While we know many factors contribute to drug abuse, OBN is committed to reducing the availability of illegal drugs in Oklahoma. Our agency works to eradicate illegal drugs in Oklahoma by enforcing drug laws, administering statewide programs, and providing continual outreach to our stakeholders – lawmakers, law enforcement, public health officials, and the citizens of Oklahoma. OBN agents enforce drug laws by utilizing aggressive investigative methods and administering statewide drug diversion programs. The Prescription Drug Monitoring Program is a valuable tool for practitioners, pharmacists, and law enforcement in the prevention and detection of the diversion and abuse of pharmaceutical controlled substances. As a service to our communities, OBN also administers the Safe Trips for Scripts Drug Prevention Program. This program provides citizens a safe way to discard unwanted medications by disposing of them in one of our 177 take back boxes located around the state. This drug threat assessment was created to help provide officials and citizens with helpful information about drug threats across our state. We will continue to collaborate with other agencies and the citizens to work toward a safer and healthier Oklahoma. -

Opioid Powders Page: 1 of 9
Federal Employee Program® 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.64 Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Analgesics and Anesthetics Original Policy Date: October 20, 2017 Subject: Opioid Powders Page: 1 of 9 Last Review Date: March 13, 2020 Opioid Powders Description Buprenorphine Powder, Butorphanol Powder, Codeine Powder, Hydrocodone Powder, Hydromorphone Powder, Levorphanol Powder, Meperidine Powder, Methadone Powder, Morphine Powder, Oxycodone Powder, Oxymorphone Powder Background Pharmacy compounding is an ancient practice in which pharmacists combine, mix or alter ingredients to create unique medications that meet specific needs of individual patients. Some examples of the need for compounding products would be: the dosage formulation must be changed to allow a person with dysphagia (trouble swallowing) to have a liquid formulation of a commercially available tablet only product, or to obtain the exact strength needed of the active ingredient, to avoid ingredients that a particular patient has an allergy to, or simply to add flavoring to medication to make it more palatable. Buprenorphine, butorphanol, codeine, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxycodone, and oxymorphone powders are opioid drugs that are used for pain control. The intent of the criteria is to provide coverage consistent with product labeling, FDA guidance, standards of medical practice, evidence-based drug information, and/or published guidelines. Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, and because of the greater risks of overdose and death (1-15). Regulatory Status 5.70.64 Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Analgesics and Anesthetics Original Policy Date: October 20, 2017 Subject: Opioid Powders Page: 2 of 9 FDA-approved indications: 1. -
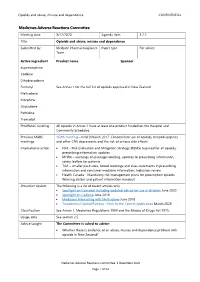
3.2.2 Opioids and Abuse, Misuse and Dependence
Opioids and abuse, misuse and dependence CONFIDENTIAL Medicines Adverse Reactions Committee Meeting date 3/12/2020 Agenda item 3.2.2 Title Opioids and abuse, misuse and dependence Submitted by Medsafe Pharmacovigilance Paper type For advice Team Active ingredient Product name Sponsor Buprenorphine Codeine Dihydrocodeine Fentanyl See Annex 1 for the full list of opioids approved in New Zealand Methadone Morphine Oxycodone Pethidine Tramadol PHARMAC funding All opioids in Annex 1 have at least one product funded on the Hospital and Community Schedules. Previous MARC 169th meeting – held 9 March 2017: Concomitant use of opioids, benzodiazepines meetings and other CNS depressants and the risk of serious side effects International action • FDA - Risk Evaluation and Mitigation Strategy (REMS) required for all opioids; prescribing information updates • MHRA – warnings on package labelling, updates to prescribing information, safety leaflets for patients • TGA – smaller pack sizes, boxed warnings and class statements in prescribing information and consumer medicine information, indication review • Health Canada – Mandatory risk management plans for prescription opioids, Warning sticker and patient information handout Prescriber Update The following is a list of recent articles only. • Spotlight on tramadol including updated advice for use in children June 2020 • Spotlight on Codeine June 2018 • Medicines Interacting with Methadone June 2018 • Transdermal Opioid Patches - Stick to the Correct Application March 2020 Classification See Annex 1. Medicines -

Patterns of Opioid Prescribing in Minnesota: 2012 and 2015
ISSUE BRIEF | APRIL, 2018 Patterns of Opioid Prescribing in Minnesota: 2012 and 2015 Introduction Key Findings: Opioids are a class of drugs that include prescription • Overall rates of opioid prescribing declined in opioid medications for pain relief —such as oxycodone Minnesota from 2012 to 2015, but the morphine (OxyContin®), hydrocodone (Vicodin®), codeine, morphine, milligram equivalents (MME) per prescription and fentanyl—as well as illicitly produced drugs like heroin increased. and fentanyl-related substances (also called fentanyl analogs).1 While prescription opioids play a role in the • Medicare and Medicaid, where eligibility is determined management of some types of severe acute, cancer-related by age, disability status, and/or income, covered and end-of-life pain, increased opioid use since 1990, approximately one-third of Minnesotans with general including for chronic pain unrelated to cancer, has resulted health coverage and accounted for two-thirds of opioid in sharply rising opioid addiction and overdoses, as well as prescriptions filled in 2015. increased healthcare utilization and costs. Recent Centers • Nearly one in three Minnesotans with an opioid for Disease Control and Prevention (CDC) guidelines point prescription in 2015 had multiple prescribers. out the limitations of the evidence base in support of opioid therapy for pain, recommend non-opioid therapy • In both 2012 and 2015, 6 in 10 opioid prescriptions for chronic pain, and emphasize the risks associated with were filled within 15 days of the patient’s last medical opioid therapy.2 In Minnesota, opioids—both prescription visit; however, 1 in 10 opioid prescriptions were filled and illicit—were responsible for 336 overdose deaths without a medical visit in the past 90 days, suggesting in 2015, more than a six-fold increase since 2000.3 In closer patient-prescriber communication or opioid 2016, opioid use accounted for 395 overdose deaths in oversight may be needed in some cases. -

Perioperative Immunosuppression and Risk of Cancer Progression: the Impact of Opioids on Pain Management
Hindawi Pain Research and Management Volume 2018, Article ID 9293704, 8 pages https://doi.org/10.1155/2018/9293704 Review Article Perioperative Immunosuppression and Risk of Cancer Progression: The Impact of Opioids on Pain Management Renata Zaja˛czkowska ,1 Wojciech Leppert,2 Joanna Mika ,3 Magdalena Kocot-Ke˛pska,4 Jarosław Woron´ ,1 Anna Wrzosek ,1 and Jerzy Wordliczek 1 1Department of Interdisciplinary Intensive Care, Jagiellonian University Medical College, Krako´w, Poland 2Department of Palliative Medicine, Poznan University of Medical Sciences, Poznan, Poland 3Department of Pain Pharmacology, Institute of Pharmacology, Polish Academy of Sciences, Krak´ow, Poland 4Department of Pain Research and Treatment, Jagiellonian University Medical College, Krako´w, Poland Correspondence should be addressed to Anna Wrzosek; [email protected] Received 24 May 2018; Revised 5 August 2018; Accepted 19 August 2018; Published 19 September 2018 Academic Editor: Massimiliano Valeriani Copyright © 2018 Renata Zaja˛czkowska et al. -is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Opioids comprise an important group of drugs used in cancer pain pharmacotherapy. In recent years, more and more studies have emerged indicating the potentially immunosuppressive effects of opioid analgesics and their serious consequences, including the risk of cancer progression. -e identification of these risks has prompted a search for other effective, and most importantly, safer methods of perioperative analgesic management. Regional analgesia techniques, which allow for a significant reduction in opioid dosing and thus diminish the risk of immunosuppression associated with these drugs, seem to offer substantial hope in this respect. -

Pre - PA Allowance Age 12 Years of Age Or Older – Ultracet (Tramadol and Acetaminophen) and Codeine/APAP Products
OPIOID IR COMBO DRUGS Apadaz* (benzhydrocodone-acetaminophen), Codeine-acetaminophen, Dvorah* (dihydrocodeine-caffeine-acetaminophen*), Hydrocodone-acetaminophen, Hydrocodone- acetaminophen solution 10-325mg*, Hydrocodone-ibuprofen, Nalocet* (oxycodone- acetaminophen*), Oxycodone-acetaminophen, Oxycodone-aspirin, Oxycodone-ibuprofen, Primlev*/Prolate* (oxycodone-acetaminophen*), Tramadol-acetaminophen, Trezix (dihydrocodeine-caffeine-acetaminophen) *Prior authorization for certain non-covered formulations applies only to formulary exceptions Pre - PA Allowance Age 12 years of age or older – Ultracet (tramadol and acetaminophen) and Codeine/APAP products Quantity Patients 18 years or older will be able to fill the Pre-PA Allowance after they have filled an initial 7 day supply of IR opioid therapy or if they have been on IR or ER opioid therapy in the last 180 days Patients age 17 and under will require a PA after they have filled a 3 day supply of the Pre- PA Allowance Patients with opioid addiction treatment or methadone in the last 30 days will not be eligible for Pre-PA Allowance Immediate Release Tablets or Capsules ≤ 50 MME/day Medication Strength Quantity Limit Codeine/APAP soln 120-12 mg/5 mL Hydrocodone/APAP soln 7.5/325 mg/15 mL 5400 mL per 90 days Hydrocodone/APAP elixir 10/300 mg/15 mL Oxycodone/APAP soln 5-325 mg/5 mL 3000 mL per 90 days Hydrocodone/ibuprofen 5/200 mg, 7.5/200 mg, 10/200 mg 270 units per 90 days Oxycodone/ibuprofen 5/400 mg Oxycodone/APAP 10/325 mg Codeine/APAP 60/300 mg Hydrocodone/APAP 7.5/300 mg, 7.5/325 -

Opioid Prescription Drug Monitoring Surveillance Report 2015
Opioid Prescription Drug Monitoring Surveillance Report 2015 Department of Health and Human Services Division of Public Health Opioid Prescription Drug Monitoring Surveillance Report Release date: November 28, 2016 Report prepared by: Megan Key, BS Jamie S. White, MPH Melody Law, MD, MPH Olivia Kasirye, MD, MS Sacramento County Department of Health and Human Services Public Health Division Disease Control and Epidemiology Unit 7001‐A East Parkway, Suite 600 Sacramento, CA 95823 Phone: 916‐875‐5881 TTY: 877‐835‐2929 Website: www.scph.com i Opioid Prescription Drug Monitoring Surveillance Report Introduction Contents INTRODUCTION ....................................................................................................................................................................... 1 The Opioid Epidemic ........................................................................................................................................................... 1 Controlled Substance Utilization Review and Evaluation System (CURES) ........................................................................ 1 Prescriber Guidelines .......................................................................................................................................................... 2 Purpose ............................................................................................................................................................................... 2 Acknowledgments .............................................................................................................................................................. -

The Main Tea Eta a El Mattitauli Mali Malta
THE MAIN TEA ETA USA 20180169172A1EL MATTITAULI MALI MALTA ( 19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2018 /0169172 A1 Kariman (43 ) Pub . Date : Jun . 21 , 2018 ( 54 ) COMPOUND AND METHOD FOR A61K 31/ 437 ( 2006 .01 ) REDUCING APPETITE , FATIGUE AND PAIN A61K 9 / 48 (2006 .01 ) (52 ) U . S . CI. (71 ) Applicant : Alexander Kariman , Rockville , MD CPC . .. .. .. .. A61K 36 / 74 (2013 .01 ) ; A61K 9 / 4825 (US ) (2013 . 01 ) ; A61K 31/ 437 ( 2013 . 01 ) ; A61K ( 72 ) Inventor: Alexander Kariman , Rockville , MD 31/ 4375 (2013 .01 ) (US ) ( 57 ) ABSTRACT The disclosed invention generally relates to pharmaceutical (21 ) Appl . No. : 15 /898 , 232 and nutraceutical compounds and methods for reducing appetite , muscle fatigue and spasticity , enhancing athletic ( 22 ) Filed : Feb . 16 , 2018 performance , and treating pain associated with cancer, trauma , medical procedure , and neurological diseases and Publication Classification disorders in subjects in need thereof. The disclosed inven ( 51 ) Int. Ci. tion further relates to Kratom compounds where said com A61K 36 / 74 ( 2006 .01 ) pound contains at least some pharmacologically inactive A61K 31/ 4375 ( 2006 .01 ) component. pronuPatent Applicationolan Publication manu saJun . decor21, 2018 deSheet les 1 of 5 US 2018 /0169172 A1 reta Mitragynine 7 -OM - nitragynine *** * *momoda W . 00 . Paynantheine Speciogynine **** * * * ! 1000 co Speclociliatine Corynartheidine Figure 1 Patent Application Publication Jun . 21, 2018 Sheet 2 of 5 US 2018 /0169172 A1 -

Methadone & EDDP by Liquid/Liquid Extraction and Gas
OFFICE OF CHIEF MEDICAL EXAMINER CITY OF NEW YORK METHADONE & EDDP BY LIQUID/LIQUID EXTRACTION AND GAS CHROMATOGRAPHY/MASS SPECTROMETRY (SELECTIVE ION MONITORING) PRINCIPLE Methadone (Symoron, Dolophine, Amidone, Methadose, Physeptone, Heptadon and many other names) is a synthetic opioid developed in Germany in 1937. It is used medically as an analgesic and also as a maintenance anti-addictive and reductive preparation for use by patients with opioid dependence. Because it is structurally an acyclic analog of morphine, methadone also acts upon the same opioid receptors, and thus has many of the same effects. Because of its long duration of action, strong analgesic effects, methadone is also used in managing severe chronic pain. Eli Lilly and Company introduced methadone into the United States in 1947. The abuse of methadone results in approximately 5,000 overdose deaths per year in the United States. Methadone is mainly used in the treatment of opioid dependence. It has cross-tolerance with other opioids including morphine, codeine and heroin, and offers very similar effects but with a longer duration. Oral doses of methadone can stabilize patients by mitigating opioid withdrawal syndrome or making it more tolerable. Higher doses can block the euphoric effects of heroin and morphine. Methadone is produced and distributed by a number of pharmaceutical companies. The racemic hydrochloride is the only form available in most countries, such as the Netherlands, Belgium, France and in the United States (as of March 2008). The dextrorotary enantiomer of methadone, dextromethadone, is an NMDA antagonist rather than an opiate agonist. As a result, methadone medications used for opiate addiction occasionally will contain only levomethadone, the levorotary enantiomer. -

The Inhibition of Enkephalin Catabolism by Dual Enkephalinase Inhibitor: a Novel Possible Therapeutic Approach for Opioid Use Disorders
Alvarez-Perez Beltran (Orcid ID: 0000-0001-8033-3136) Maldonado Rafael (Orcid ID: 0000-0002-4359-8773) THE INHIBITION OF ENKEPHALIN CATABOLISM BY DUAL ENKEPHALINASE INHIBITOR: A NOVEL POSSIBLE THERAPEUTIC APPROACH FOR OPIOID USE DISORDERS ALVAREZ-PEREZ Beltran1*, PORAS Hervé 2*, MALDONADO Rafael1 1 Laboratory of Neuropharmacology, Department of Experimental and Health Sciences, Universitat Pompeu Fabra, Barcelona Biomedical Research Park, c/Dr Aiguader 88, 08003 Barcelona, Spain, 2 Pharmaleads, Paris BioPark, 11 Rue Watt, 75013 Paris, France *Both authors participated equally to the manuscript Correspondence: Rafael Maldonado, Laboratori de Neurofarmacologia, Universitat Pompeu Fabra, Parc de Recerca Biomèdica de Barcelona (PRBB), c/Dr. Aiguader, 88, 08003 Barcelona, Spain. E-mail: [email protected] ABSTRACT Despite the increasing impact of opioid use disorders on society, there is a disturbing lack of effective medications for their clinical management. An interesting innovative strategy to treat these disorders consists in the protection of endogenous opioid peptides to activate opioid receptors, avoiding the classical opioid-like side effects. Dual Enkephalinase Inhibitors (DENKIs) physiologically activate the endogenous opioid system by inhibiting the enzymes responsible for the breakdown of enkephalins, protecting endogenous enkephalins, increasing their half-lives and physiological actions. The activation of opioid receptors by the increased enkephalin levels, and their well-demonstrated safety, suggest that DENKIs could represent a novel analgesic therapy and a possible effective treatment for acute opioid withdrawal, as well as a promising alternative to opioid substitution therapy minimizing side effects. This new pharmacological class of compounds could bring effective and safe medications avoiding the This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. -

Effects of Medication-Assisted Treatment (MAT) on Functional Outcomes Among Patients with Opioid Use Disorder (OUD)
NATIONAL DEFENSE RESEARCH INSTITUTE Effects of Medication- Assisted Treatment (MAT) for Opioid Use Disorder on Functional Outcomes A Systematic Review Margaret A. Maglione, Laura Raaen, Christine Chen, Gulrez Shah Azhar, Nima Shahidinia, Mimi Shen, Ervant J. Maksabedian Hernandez, Roberta M. Shanman, Susanne Hempel Prepared for the Office of the Secretary of Defense Approved for public release; distribution unlimited For more information on this publication, visit www.rand.org/t/RR2108 Published by the RAND Corporation, Santa Monica, Calif. © Copyright 2018 RAND Corporation R® is a registered trademark. Limited Print and Electronic Distribution Rights This document and trademark(s) contained herein are protected by law. This representation of RAND intellectual property is provided for noncommercial use only. Unauthorized posting of this publication online is prohibited. Permission is given to duplicate this document for personal use only, as long as it is unaltered and complete. Permission is required from RAND to reproduce, or reuse in another form, any of its research documents for commercial use. For information on reprint and linking permissions, please visit www.rand.org/pubs/permissions. The RAND Corporation is a research organization that develops solutions to public policy challenges to help make communities throughout the world safer and more secure, healthier and more prosperous. RAND is nonprofit, nonpartisan, and committed to the public interest. RAND’s publications do not necessarily reflect the opinions of its research clients and sponsors. Support RAND Make a tax-deductible charitable contribution at www.rand.org/giving/contribute www.rand.org Preface Over the past two decades, the U.S. Department of Defense (DoD) has invested unparalleled resources into developing effective treatments for military-related psychological health conditions.