Opioid Powders Page: 1 of 9
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pre - PA Allowance Age 12 Years of Age Or Older – Ultracet (Tramadol and Acetaminophen) and Codeine/APAP Products
OPIOID IR COMBO DRUGS Apadaz* (benzhydrocodone-acetaminophen), Codeine-acetaminophen, Dvorah* (dihydrocodeine-caffeine-acetaminophen*), Hydrocodone-acetaminophen, Hydrocodone- acetaminophen solution 10-325mg*, Hydrocodone-ibuprofen, Nalocet* (oxycodone- acetaminophen*), Oxycodone-acetaminophen, Oxycodone-aspirin, Oxycodone-ibuprofen, Primlev*/Prolate* (oxycodone-acetaminophen*), Tramadol-acetaminophen, Trezix (dihydrocodeine-caffeine-acetaminophen) *Prior authorization for certain non-covered formulations applies only to formulary exceptions Pre - PA Allowance Age 12 years of age or older – Ultracet (tramadol and acetaminophen) and Codeine/APAP products Quantity Patients 18 years or older will be able to fill the Pre-PA Allowance after they have filled an initial 7 day supply of IR opioid therapy or if they have been on IR or ER opioid therapy in the last 180 days Patients age 17 and under will require a PA after they have filled a 3 day supply of the Pre- PA Allowance Patients with opioid addiction treatment or methadone in the last 30 days will not be eligible for Pre-PA Allowance Immediate Release Tablets or Capsules ≤ 50 MME/day Medication Strength Quantity Limit Codeine/APAP soln 120-12 mg/5 mL Hydrocodone/APAP soln 7.5/325 mg/15 mL 5400 mL per 90 days Hydrocodone/APAP elixir 10/300 mg/15 mL Oxycodone/APAP soln 5-325 mg/5 mL 3000 mL per 90 days Hydrocodone/ibuprofen 5/200 mg, 7.5/200 mg, 10/200 mg 270 units per 90 days Oxycodone/ibuprofen 5/400 mg Oxycodone/APAP 10/325 mg Codeine/APAP 60/300 mg Hydrocodone/APAP 7.5/300 mg, 7.5/325 -

The Inhibition of Enkephalin Catabolism by Dual Enkephalinase Inhibitor: a Novel Possible Therapeutic Approach for Opioid Use Disorders
Alvarez-Perez Beltran (Orcid ID: 0000-0001-8033-3136) Maldonado Rafael (Orcid ID: 0000-0002-4359-8773) THE INHIBITION OF ENKEPHALIN CATABOLISM BY DUAL ENKEPHALINASE INHIBITOR: A NOVEL POSSIBLE THERAPEUTIC APPROACH FOR OPIOID USE DISORDERS ALVAREZ-PEREZ Beltran1*, PORAS Hervé 2*, MALDONADO Rafael1 1 Laboratory of Neuropharmacology, Department of Experimental and Health Sciences, Universitat Pompeu Fabra, Barcelona Biomedical Research Park, c/Dr Aiguader 88, 08003 Barcelona, Spain, 2 Pharmaleads, Paris BioPark, 11 Rue Watt, 75013 Paris, France *Both authors participated equally to the manuscript Correspondence: Rafael Maldonado, Laboratori de Neurofarmacologia, Universitat Pompeu Fabra, Parc de Recerca Biomèdica de Barcelona (PRBB), c/Dr. Aiguader, 88, 08003 Barcelona, Spain. E-mail: [email protected] ABSTRACT Despite the increasing impact of opioid use disorders on society, there is a disturbing lack of effective medications for their clinical management. An interesting innovative strategy to treat these disorders consists in the protection of endogenous opioid peptides to activate opioid receptors, avoiding the classical opioid-like side effects. Dual Enkephalinase Inhibitors (DENKIs) physiologically activate the endogenous opioid system by inhibiting the enzymes responsible for the breakdown of enkephalins, protecting endogenous enkephalins, increasing their half-lives and physiological actions. The activation of opioid receptors by the increased enkephalin levels, and their well-demonstrated safety, suggest that DENKIs could represent a novel analgesic therapy and a possible effective treatment for acute opioid withdrawal, as well as a promising alternative to opioid substitution therapy minimizing side effects. This new pharmacological class of compounds could bring effective and safe medications avoiding the This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. -

Effects of Medication-Assisted Treatment (MAT) on Functional Outcomes Among Patients with Opioid Use Disorder (OUD)
NATIONAL DEFENSE RESEARCH INSTITUTE Effects of Medication- Assisted Treatment (MAT) for Opioid Use Disorder on Functional Outcomes A Systematic Review Margaret A. Maglione, Laura Raaen, Christine Chen, Gulrez Shah Azhar, Nima Shahidinia, Mimi Shen, Ervant J. Maksabedian Hernandez, Roberta M. Shanman, Susanne Hempel Prepared for the Office of the Secretary of Defense Approved for public release; distribution unlimited For more information on this publication, visit www.rand.org/t/RR2108 Published by the RAND Corporation, Santa Monica, Calif. © Copyright 2018 RAND Corporation R® is a registered trademark. Limited Print and Electronic Distribution Rights This document and trademark(s) contained herein are protected by law. This representation of RAND intellectual property is provided for noncommercial use only. Unauthorized posting of this publication online is prohibited. Permission is given to duplicate this document for personal use only, as long as it is unaltered and complete. Permission is required from RAND to reproduce, or reuse in another form, any of its research documents for commercial use. For information on reprint and linking permissions, please visit www.rand.org/pubs/permissions. The RAND Corporation is a research organization that develops solutions to public policy challenges to help make communities throughout the world safer and more secure, healthier and more prosperous. RAND is nonprofit, nonpartisan, and committed to the public interest. RAND’s publications do not necessarily reflect the opinions of its research clients and sponsors. Support RAND Make a tax-deductible charitable contribution at www.rand.org/giving/contribute www.rand.org Preface Over the past two decades, the U.S. Department of Defense (DoD) has invested unparalleled resources into developing effective treatments for military-related psychological health conditions. -

Opioid-Containing Cough and Cold Products - Assessing the Potential Risk of Opioid Use Disorder and Related Harms in Children and Adolescents
Summary Safety Review –Opioid-containing cough and cold products - Assessing the potential risk of opioid use disorder and related harms in children and adolescents Product: Cough and cold products containing opioids (including codeine, hydrocodone or normethadone) Potential Safety Issue: Opioid use disorders and related harms in children and adolescents Key Messages • The growing number of overdoses and harms caused by opioids is a major public health concern in Canada. Cough and cold products containing opioids (including codeine, hydrocodone or normethadone) are authorized for sale in Canada to treat cough and cold symptoms in adults and children. • Health Canada reviewed the risk of opioid use disorder and related harms from these products after the United States Food and Drug Administration (US FDA) advised against using these products in children and adolescents in 2018. Health Canada’s safety review found limited evidence to link opioid-containing cough and cold products with opioid use disorders and related harms in children and adolescents. • These products are linked to other known harms (i.e., breathing problems), and there is limited evidence to support the effectiveness of these products in children and adolescents. There are other products available in Canada to help relieve the symptoms of cough and cold in children. • Therefore, Health Canada, as a precautionary measure, is advising Canadians against the use of these products among children and adolescents under 18 years of age. Health Canada will notify the manufacturers to update the product safety information of opioid- containing cough and cold products to limit the recommended age of use (indication) to adults only, 18 years of age and older. -

List of Narcotic Drugs Under International Control
International Narcotics Control Board Yellow List Annex to Forms A, B and C 59th edition, July 2020 LIST OF NARCOTIC DRUGS UNDER INTERNATIONAL CONTROL Prepared by the INTERNATIONAL NARCOTICS CONTROL BOARD* Vienna International Centre P.O. Box 500 A-1400 Vienna, Austria Internet address: http://www.incb.org/ in accordance with the Single Convention on Narcotic Drugs, 1961** Protocol of 25 March 1972 amending the Single Convention on Narcotic Drugs, 1961 * On 2 March 1968, this organ took over the functions of the Permanent Central Narcotics Board and the Drug Supervisory Body, r etaining the same secretariat and offices. ** Subsequently referred to as “1961 Convention”. V.20-03697 (E) *2003697* Purpose The Yellow List contains the current list of narcotic drugs under international control and additional relevant information. It has been prepared by the International Narcotics Control Board to assist Governments in completing the annual statistical reports on narcotic drugs (Form C), the quarterly statistics of imports and exports of narcotic drugs (Form A) and the estimates of annual requirements for narcotic drugs (Form B) as well as related questionnaires. The Yellow List is divided into four parts: Part 1 provides a list of narcotic drugs under international control in the form of tables and is subdivided into three sections: (1) the first section includes the narcotic drugs listed in Schedule I of the 1961 Convention as well as intermediate opiate raw materials; (2) the second section includes the narcotic drugs listed in Schedule II of the 1961 Convention; and (3) the third section includes the narcotic drugs listed in Schedule IV of the 1961 Convention. -

Modulation of Opioid Transport at the Blood-Brain Barrier by Altered ATP-Binding Cassette (ABC) Transporter Expression and Activity
pharmaceutics Review Modulation of Opioid Transport at the Blood-Brain Barrier by Altered ATP-Binding Cassette (ABC) Transporter Expression and Activity Junzhi Yang 1, Bianca G. Reilly 2, Thomas P. Davis 1,2 and Patrick T. Ronaldson 1,2,* 1 Department of Pharmacology and Toxicology, College of Pharmacy, University of Arizona, 1295 N. Martin St., P.O. Box 210207, Tucson, AZ 85721, USA; [email protected] (J.Y.); [email protected] (T.P.D.) 2 Department of Pharmacology, College of Medicine, University of Arizona, 1501 N. Campbell Ave, P.O. Box 245050, Tucson, AZ 85724-5050, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-520-626-2173 Received: 19 September 2018; Accepted: 16 October 2018; Published: 18 October 2018 Abstract: Opioids are highly effective analgesics that have a serious potential for adverse drug reactions and for development of addiction and tolerance. Since the use of opioids has escalated in recent years, it is increasingly important to understand biological mechanisms that can increase the probability of opioid-associated adverse events occurring in patient populations. This is emphasized by the current opioid epidemic in the United States where opioid analgesics are frequently abused and misused. It has been established that the effectiveness of opioids is maximized when these drugs readily access opioid receptors in the central nervous system (CNS). Indeed, opioid delivery to the brain is significantly influenced by the blood-brain barrier (BBB). In particular, ATP-binding cassette (ABC) transporters that are endogenously expressed at the BBB are critical determinants of CNS opioid penetration. In this review, we will discuss current knowledge on the transport of opioid analgesic drugs by ABC transporters at the BBB. -

Hydromorphone Hydrochloride) CS-II WARNING: DILAUDID ORAL LIQUID and DILAUDID TABLETS CONTAIN HYDROMORPHONE, WHICH IS a POTENT SCHEDULE II CONTROLLED OPIOID AGONIST
DILAUDID® ORAL LIQUID and DILAUDID® TABLETS (hydromorphone hydrochloride) CS-II WARNING: DILAUDID ORAL LIQUID AND DILAUDID TABLETS CONTAIN HYDROMORPHONE, WHICH IS A POTENT SCHEDULE II CONTROLLED OPIOID AGONIST. SCHEDULE II OPIOID AGONISTS, INCLUDING MORPHINE, OXYMORPHONE, OXYCODONE, FENTANYL, AND METHADONE, HAVE THE HIGHEST POTENTIAL FOR ABUSE AND RISK OF PRODUCING RESPIRATORY DEPRESSION. ALCOHOL, OTHER OPIOIDS AND CENTRAL NERVOUS SYSTEM DEPRESSANTS (SEDATIVE-HYPNOTICS) POTENTIATE THE RESPIRATORY DEPRESSANT EFFECTS OF HYDROMORPHONE, INCREASING THE RISK OF RESPIRATORY DEPRESSION THAT MIGHT RESULT IN DEATH. DESCRIPTION Proprietary name: DILAUDID ORAL LIQUID Established name: hydromorphone hydrochloride Route of administration: ORAL (C38288) Active ingredients (moiety): hydromorphone hydrochloride (hydromorphone) # Strength Form Inactive ingredients 1 5 MILLIGRAM LIQUID purified water, methylparaben, propylparaben, sucrose, glycerin, sodium (C42953) metabisulfite Proprietary name: DILAUDID TABLETS Established name: hydromorphone hydrochloride Route of administration: ORAL (C38288) Active ingredients (moiety): hydromorphone hydrochloride (hydromorphone) # Strength Form Inactive ingredients 1 2 MILLIGRAM TABLET D&C red #30 Lake dye, D&C yellow #10 Lake dye, lactose, magnesium (C42998) stearate, sodium metabisulfite 2 4 MILLIGRAM TABLET D&C yellow #10 Lake dye, lactose, magnesium stearate, sodium metabisulfite (C42998) 3 8 MILLIGRAM TABLET lactose anhydrous, magnesium stearate, sodium metabisulfite (C42998) DILAUDID (hydromorphone hydrochloride), a hydrogenated ketone of morphine, is an opioid analgesic. The chemical name of DILAUDID (hydromorphone hydrochloride) is 4,5α-epoxy-3- hydroxy-17-methylmorphinan-6-one hydrochloride. The structural formula is: M.W. 321.8 Each 5 mL (1 teaspoon) of DILAUDID ORAL LIQUID contains 5 mg of hydromorphone hydrochloride. In addition, other ingredients include purified water, methylparaben, propylparaben, sucrose, and glycerin. DILAUDID ORAL LIQUID may contain traces of sodium metabisulfite. -
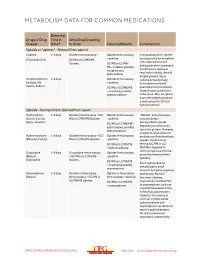
Metabolism Data for Common Medications
METABOLISM DATA FOR COMMON MEDICATIONS Detection Drugs or Drug Time in Urine Drug Screening Classes Urine* to Order Expected Results Consideration Opioids or “opiates” – Natural (from opium) Codeine 1–3 days Opiates Immunoassay + Opiates Immunoassay Immunoassays for “opiates” are responsive for morphine (Tylenol #2/3/4) GC/MS or LC/MS/MS – positive and codeine but do not Opiates GC/MS or LC/MS/ distinguish which is present. MS – codeine, possibly Confirmatory testing is morphine and required to reliably identify hydrocodone drug(s) present. Since Morphine (Avinza, 1–3 days Opiates Immunoassay codeine is metabolized Embeda, MS – positive to morphine and small Contin, Kadian) GC/MS or LC/MS/MS quantities to hydrocodone, – morphine, possibly these drugs may be found hydromorphone in the urine. Also, morphine may metabolize to produce a small amount (<10%) of hydromorphone. Opioids – Semisynthetic (derived from opium Hydrocodone 1–3 days Opiates Immunoassay + GC/ Opiates Immunoassay “Opiates” immunoassays (Lorcet, Lortab, MS or LC/MS/MS Opiates – positive may also detect Norco, Vicodin) semisynthetic opioids GC/MS or LC/MS/MS – depending on their cross- hydrocodone, possibly reactivity pattern. However, hydromorphone a negative result does not Hydromorphone 1–3 days Opiates Immunoassay + GC/ Opiates Immunoassay exclude use of semisynthetic (Dilaudid, Exalgo) MS or LC/MS/MS Opiates –positive opioids. Confirmatory GC/MS or LC/MS/MS testing (GC/MS or LC/ – hydromorphone MS/MS) is required to verify compliance with the Oxycodone 1–3 days Oxycodone Immunoassay Opiates Immunoassay prescribed semisynthetic (Roxicet, + GC/MS or LC/MS/MS –positive opioid(s). OxyContin) Opiates GC/MS or LC/MS/MS Since hydrocodone is – oxycodone possibly metabolized in small oxymorphone amounts to hydromorphone, Oxymorphone 1–3 days Opiates or Oxycodone Opiates or Oxycodone both may be found in (Opana) Immunoassay + GC/MS or Immunoassay – positive the urine. -

Use of Chronic Opioid Therapy in Chronic Noncancer Pain CHRONIC NONCANCER PAINNONCANCER CHRONIC Evidence Review
CLINICAL GUIDELINE FOR THE USE OF CHRONIC OPIOID THERAPY IN CLINICAL GUIDELINE FOR THE USE OF CHRONIC OPIOID THERAPY IN GUIDELINE FOR THE Use of Chronic Opioid Therapy in Chronic Noncancer Pain CHRONIC NONCANCER PAIN Evidence Review The American Pain Society in Conjunction with The American Academy of Pain Medicine EVIDENCE REVIEW APS-AAPM Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain TABLE OF CONTENTS Page Introduction 1 Purpose of evidence review ...................................................................... 1 Background 1 Previous guidelines ................................................................................... 2 Scope of evidence review 3 Key questions............................................................................................ 3 Populations................................................................................................ 7 Interventions.............................................................................................. 8 Outcomes.................................................................................................. 8 Conflict of interest............................................................................................. 10 Methods 10 Literature search and strategy................................................................... 10 Inclusion and exclusion criteria.................................................................. 11 Data extraction and synthesis .................................................................. -

The Effect of Preoperative Acetaminophen/Hydrocodone on the Efficacy of the Inferior Alveolar Nerve Block in Patients with Symptomatic Irreversible Pulpitis
THE EFFECT OF PREOPERATIVE ACETAMINOPHEN/HYDROCODONE ON THE EFFICACY OF THE INFERIOR ALVEOLAR NERVE BLOCK IN PATIENTS WITH SYMPTOMATIC IRREVERSIBLE PULPITIS A Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Graduate School of The Ohio State University By Spencer C. Fullmer D.D.S. Graduate Program in Dentistry The Ohio State University 2012 Master’s Examination Committee: Melissa Drum D.D.S. M.S., Advisor Al Reader D.D.S. M.S. John Nusstein D.D.S. M.S. F. Michael Beck D.D.S. M.A. Copyright by Spencer C. Fullmer D.D.S. 2012 ABSTRACT The inferior alveolar nerve block (IANB) is frequently used as a mandibular injection technique for achieving local anesthesia for endodontic treatment. Successful anesthesia, however, is not always achieved with the IANB. Studies have suggested that preoperative medications may increase the ability of clinicians to achieve profound pulpal anesthesia in conjunction with local anesthesia techniques. Acetaminophen/hydrocodone combination medications have been shown to effectively manage pain in a variety of dental pain models. The purpose of this prospective, randomized, double-blind, placebo-controlled study was to determine the effect of the administration of acetaminophen/hydrocodone on the efficacy of the IANB in patients with symptomatic irreversible pulpitis. One hundred emergency patients diagnosed with symptomatic irreversible pulpitis of a mandibular posterior tooth randomly received (in a double-blind manner) either 10 mg hydrocodone and 1000 mg acetaminophen (divided into four capsules) or an identical placebo 60 minutes before the administration of a conventional IANB. Fifteen minutes after administration of the IANB, profound lip numbness was confirmed, and endodontic access was initiated. -

Replacement of Current Opioid Drugs Focusing on MOR-Related Strategies
JPT-107519; No of Pages 17 Pharmacology & Therapeutics xxx (2020) xxx Contents lists available at ScienceDirect Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/pharmthera Replacement of current opioid drugs focusing on MOR-related strategies Jérôme Busserolles a,b, Stéphane Lolignier a,b, Nicolas Kerckhove a,b,c, Célian Bertin a,b,c, Nicolas Authier a,b,c, Alain Eschalier a,b,⁎ a Université Clermont Auvergne, INSERM, CHU, NEURO-DOL Pharmacologie Fondamentale et Clinique de la douleur, F-63000 Clermont-Ferrand, France b Institut ANALGESIA, Faculté de Médecine, F-63000 Clermont-Ferrand, France c Observatoire Français des Médicaments Antalgiques (OFMA), French monitoring centre for analgesic drugs, CHU, F-63000 Clermont-Ferrand, France article info abstract Available online xxxx The scarcity and limited risk/benefit ratio of painkillers available on the market, in addition to the opioid crisis, warrant reflection on new innovation strategies. The pharmacopoeia of analgesics is based on products that are often old and derived from clinical empiricism, with limited efficacy or spectrum of action, or resulting in Keywords: an unsatisfactory tolerability profile. Although they are reference analgesics for nociceptive pain, opioids are sub- Analgesia ject to the same criticism. The use of opium as an analgesic is historical. Morphine was synthesized at the begin- Mu opioid receptors (MORs) ning of the 19th century. The efficacy of opioids is limited in certain painful contexts and these drugs can induce Opioid adverse side effects potentially serious and fatal adverse effects. The current North American opioid crisis, with an ever-rising number Opioid abuse and misuse of deaths by opioid overdose, is a tragic illustration of this. -

Federal Register/Vol. 85, No. 230/Monday, November 30, 2020
76604 Federal Register / Vol. 85, No. 230 / Monday, November 30, 2020 / Notices notifications disclosing all changes in Act on September 18, 2020 (85 FR DEPARTMENT OF JUSTICE membership. 58390). Drug Enforcement Administration On September 10, 2018, CONFERS Suzanne Morris, [Docket No. DEA–688E] filed its original notification pursuant to Chief, Premerger and Division Statistics, Section 6(a) of the Act. The Department Antitrust Division. Established Aggregate Production of Justice published a notice in the [FR Doc. 2020–26362 Filed 11–27–20; 8:45 am] Federal Register pursuant to Section Quotas for Schedule I and II Controlled BILLING CODE P Substances and Assessment of 6(b) of the Act on October 19, 2018 (83 Annual Needs for the List I Chemicals FR 53106). Ephedrine, Pseudoephedrine, and The last notification was filed with DEPARTMENT OF JUSTICE Phenylpropanolamine for 2021 the Department on May 1, 2020. A notice was published in the Federal Antitrust Division AGENCY: Drug Enforcement Register pursuant to Section 6(b) of the Administration, Department of Justice. Act on May 28, 2020 (85 FR 32049). Notice Pursuant to the National ACTION: Final order. Cooperative Research and Production Suzanne Morris, Act of 1993—Cooperative Research SUMMARY: This final order establishes Chief, Premerger and Division Statistics, Group on AC2AT–II the initial 2021 aggregate production Antitrust Division. quotas for controlled substances in [FR Doc. 2020–26358 Filed 11–27–20; 8:45 am] Notice is hereby given that, on schedules I and II of the Controlled Substances Act and the assessment of BILLING CODE 4410–11–P November 19, 2020, pursuant to Section 6(a) of the National Cooperative annual needs for the list I chemicals Research and Production Act of 1993, ephedrine, pseudoephedrine, and DEPARTMENT OF JUSTICE 15 U.S.C.