Optimization and Development of in Vitro Bioassays to Determine Structure-Activity Relationships for Cannabinoid Receptor 1 and Cannabinoid Receptor 2
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Cannabinoid As Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity
Cannabinoid as Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity Sudipta Baroi1, Achintya Saha2, Ritesh Bachar3 and Sitesh C Bachar4 1Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka Dhaka-1000, Bangladesh 2Department of Chemical Technology, Pharmaceutical & Fine Chemical Technology Division University of Calcutta, India 3Department of Pharmacy, School of Science and Engineering, University of Information Technology and Sciences (UITS), Dhaka-1212, Bangladesh 4Department of Pharmacy, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh (Received: April 15, 2020; Accepted: June 2, 2020; Published (web): June 28, 2020) ABSTRACT: Inhibition of aromatase (CYTP450), a key enzyme in the estrogen biosynthesis, could result in regression of estrogen-dependent tumors and even prevent the promotion of breast cancer. The present research has been designed for searching a potent chemical moiety from natural sources to inhibit aromatase enzyme, the over- functionality of which causes the breast cancer. Cannabis sativa contains a very much promising group of cannabinoids with more than 66 compounds with reported anticancer property and for the search of a target specific potent aromatase inhibitor, 61 cannabinoids from C. sativa were selected. The Structures Data File (SDF) of these ligand molecules were subjected to docking studies at the binding site of aromatase X-ray crystallographic structure based on lower resolution of the protein crystal structure and higher docking accuracy, predicted by calculating the correlation between experimental activities and Glide dock scores and compared with the standard aromatase ligand androstenedione and aromatase inhibitor fadrozole with existing drug for breast cancer treatment. The best docked pose of each ligand was selected on the basis of the highest dock score related to the binding free energies of the internal dataset compounds as compared to their observed activities. -

PDF of the Full Text
Clinical Pharmacokinetics of Cannabinoids Franjo Grotenhermen ABSTRACT. Absorption and metabolism of tetrahydrocannabinol (THC) vary as a function of route of administration. Pulmonary assimilation of in- haled THC causes a maximum plasma concentration within minutes, while psychotropic effects start within seconds to a few minutes, reach a maxi- mum after 15 to 30 minutes, and taper off within 2 to 3 hours. Following oral ingestion, psychotropic effects set in with a delay of 30 to 90 minutes, reach their maximum after 2 to 3 hours, and last for about 4 to 12 hours, de- pending on dose and specific effect. The initial volume of distribution of THC is small for a lipophilic drug, equivalent to the plasma volume of about 2.5-3 L, reflecting high protein binding of 95-99%. The steady state volume of distribution has been esti- mated to be about 100 times larger, in the range of about 3.5 L per kg of body weight. The lipophility of THC with high binding to tissue and in par- ticular to fat, the major long-term storage site, causes a change of distribu- tion pattern over time. Only about 1% of THC administered IV is found in the brain at the time of peak psychoactivity. THC crosses the placenta and small amounts penetrate into the breast milk. Metabolism of THC occurs mainly in the liver by microsomal hydroxylation and oxidation catalyzed by enzymes of the cytochrome P-450 complex. In man, the C-11 carbon is the major site attacked. Hydroxylation results in 11-hydroxy-THC (11-OH-THC) and further oxi- dation to 11-nor-9-carboxy-THC (THC-COOH), which may be glucuronated to THC-COOH beta-glucuronide. -
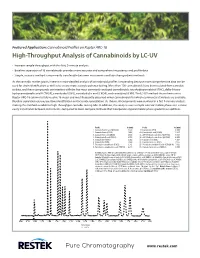
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12. -

CBD (Cannabidiol)
TRANSPORTATION RESEARCH BOARD Driving Toward the Truth - Dispelling the Myths About Cannabis Products February 10, 2021 @NASEMTRB #TRBwebinar PDH Certification The Transportation Research Board has met the standards and Information: requirements of the Registered Continuing Education Providers •1.5 Professional Development Program. Credit earned on completion Hour (PDH) – see follow-up of this program will be reported to email for instructions RCEP. A certificate of completion will •You must attend the entire be issued to participants that have registered and attended the entire webinar to be eligible to receive session. As such, it does not include PDH credits content that may be deemed or •Questions? Contact Reggie construed to be an approval or Gillum at [email protected] endorsement by RCEP. #TRBwebinar Learning Objectives 1. Identify impacts of the Farm Bill on use of THC and CBD products 2. Describe the toxicology of THC and CBD products 3. Discuss how THC and CBD products affect driving performance and crash risk #TRBwebinar TRB Standing Committee on Impairment in Transportation (ACS50) TRB Webinar: Driving Toward the Truth - Dispelling the Myths About Cannabis Products Dr. Barry K. Logan Executive Director, Center for Forensic Science Research and Education (CFSRE); Senior Vice President of Forensic Sciences, and Chief Scientist at NMS Labs Michelle Peace, Ph.D. Associate Professor and PI, Laboratory for Forensic Toxicology Research Department of Forensic Science, Virginia Commonwealth University Dr. Darrin Grondel Vice President, -

Medical Marijuana: High Expectations
Medical Marijuana: High Expectations Dr. Ally Dering-Anderson, RP University of Nebraska College of Pharmacy August 2019 What we know What we don’t Speaker Contact Information Dr. Ally Dering-Anderson, RP Clinical Associate Professor of Pharmacy University of Nebraska College of Pharmacy 986145 Medical Center College of Pharmacy Department of Pharmacy Practice Omaha, NE 68198-6145 [email protected] Speaker Disclosures Dr. Ally is on the faculty of the University of Nebraska College of Pharmacy Department of Pharmacy Practice She is the Senior Partner of BBfN, a health and safety consulting company She has no conflict in regard to medical use of cannabinoids “Off Label Use” In order to assure that there are no problems with the accreditation of this program nor problems caused for the accrediting body here goes: There are 3 approved products containing one or more chemicals found in the marijuana plant. Epidiolex (cannabidiol) Marinol Cesamet We will NOT discuss uses for these 3 approved products beyond the labeling. We will discuss cannabinoids not found in drug products currently approved by the FDA. The concept of being “off label” for these products is illogical, as there is no label to follow. Got it? Bibliography: #’s 1, 2, 3 A note from the CE clinical reviewers … “Medical marijuana is considered a controversial topic in CME. When a CME activity includes information about an approach to diagnosis or treatment that is not generally accepted, it is allowable to facilitate debate and discussion about the approach, but it is not allowable to advocate for the test or treatment, or teach clinicians how or when to use it.” Trust me, I am not advocating for any test - - in fact, I’m going to slam one. -

Recent Developments in Cannabis Chemistry
Recent Developments in Cannabis Chemistry BY ALEXANDER T. SHULGIN, Ph.D. The marijuana plant Cannabis sativa contains a bewildering Introduction array of organic chemicals. As is true with other botanic species, there are representatives of almost all chemical classes present, including mono- and sesquiterpenes, carbohy- drates, aromatics, and a variety of nitrogenous compounds. Interest in the study of this plant has centered primarily on the resinous fraction, as it is this material that is invested with the pharmacological activity that is peculiar to the plant. This resin is secreted by the female plant as a protective agent during seed ripening, although it can be found as a microscopic exudate through the aerial portions of plants of either sex. The pure resin, hashish or charas, is the most potent fraction of the plant, and has served as the source material for most of the chemical studies. The family of chemicals that has been isolated from this source has been referred to as the cannabinoid group. It is unique amongst psychotropic materials from plants in that there are no alkaloids present. The fraction is totally nitro- gen-free. Rather, the set of compounds can be considered as analogs of the parent compound cannabinol (I), a fusion product of terpene and a substituted resorcinol. Beyond the scope of this present review are such questions as the distribution of these compounds within the plant, the bo- tanic variability resulting from geographic distribution, the diversity of pharmacological action assignable to the several Reprinted from Journal of Psychedelic Drugs, vol. II, no. 1, 197 1. 397 398 Marijuana: Medical Papers distinct compounds present, and the various preparations and customs of administration. -

Crosstalk Between Cannabinoid Receptors and Epidermal
CROSSTALK BETWEEN CANNABINOID RECEPTORS AND EPIDERMAL GROWTH FACTOR RECEPTOR IN NON-SMALL CELL LUNG CANCER DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By JANANI RAVI Graduate Program in Molecular, Cellular and Developmental Biology The Ohio State University 2015 Dissertation Committee: Dr. Ramesh Ganju, Advisor Dr. Kalpana Ghoshal Dr. Xianghong Zou Dr. Sujit Basu Copyright by Janani Ravi 2015 Abstract (i) The endocannabinoid anandamide (AEA), a neurotransmitter was shown to have anti- cancer effects. Fatty acid amide hydrolase (FAAH) metabolizes AEA and decreases its anti-tumorigenic activity. In this study, we have analyzed the role of FAAH inhibition in non-small cell lung cancer (NSCLC). We have shown that FAAH and CB1 receptor which is activated by AEA are expressed in lung adenocarcinoma patient samples and NSCLC cell lines A549 and H460. Since the synthetic analogue of anandamide (Met-F- AEA) did not possess significant anti-tumorigenic effects, we used Met-F-AEA in combination with FAAH inhibitor URB597 which significantly reduced EGF (epidermal growth factor)-induced proliferative and chemotactic activities in vitro when compared to anti-tumorigenic activity of Met-F-AEA alone. Further analysis of signaling mechanisms revealed that Met-F-AEA in combination with URB597 inhibits activation of EGFR and its downstream signaling ERK, AKT and NF-kB. In addition, it inhibited MMP2 secretion and stress fiber formation. We have also shown that the Met-F-AEA in combination with URB597 induces G0/G1 cell cycle arrest by downregulating cyclin D1 and CDK4 expressions, ultimately leading to apoptosis via activation of caspase-9 and PARP. -

What Is Delta-8 THC?? Cannabinoid Chemistry 101
What is Delta-8 THC?? Cannabinoid Chemistry 101 National Conference on Weights and Measures Annual Meeting - Rochester, NY Matthew D. Curran, Ph.D. July 21, 2021 Disclaimer Just to be clear… • I am a chemist and not a lawyer so: • This presentation will not discuss the legal aspects of Δ8-THC or DEA’s current position. • This presentation will not discuss whether Δ8-THC is considered “synthetic” or “naturally occurring.” • This is not a position statement on any issues before the NCWM. • Lastly, this should only be considered a scientific sharing exercise. Florida Department of Agriculture and Consumer Services 2 Cannabis in Florida Cannabis Syllabus • What is Cannabis? • “Mother” Cannabinoid • Decarboxylation • Relationship between CBD and THC • What does “Total” mean? • Dry Weight vs. Wet Weight • What does “Delta-9” mean? • Relationship between “Delta-8” and “Delta-9” • CBD to Delta-8 THC • Cannabinoid Chemistry 202… Florida Department of Agriculture and Consumer Services 3 Cannabis Cannabis • Cannabis sativa is the taxonomic name for the plant. • The concentration of Total Δ9-Tetrahydrocannabinol (Total Δ9-THC) is critical when considering the varieties of Cannabis sativa. • Hemp – (Total Δ9-THC) 0.3% or less • Not really a controversial term, “hemp” • Marijuana/cannabis – (Total Δ9-THC) Greater than 0.3% • Controversial term, “marijuana” • Some states prohibit the use of this term whereas some states have it in their laws. • Some states use the term “cannabis.” • Not italicized • Lower case “c” Florida Department of Agriculture and -

In Silico Assessment of Drug-Like Properties of Phytocannabinoids in Cannabis Sativa
EDUCATUM JSMT Vol. 4 No. 2 (2017) ISSN 2289-7070 / eISSN 2462-2451 (1-7) https://ejournal.upsi.edu.my/journal/EDSC In Silico Assessment of Drug-Like Properties of Phytocannabinoids in Cannabis Sativa Shakinaz Desa1*, Asiah Osman2, and Richard Hyslop3 1Department of Biology, Universiti Pendidikan Sultan Idris, Malaysia, 2Natural Product Division, Forest Research Institute Malaysia, 3Department of Chemistry and Biochemistry, University of Northern Colorado, USA *Corresponding author: [email protected] Abstract This study investigated drug-like properties of phytocannabinoids in Cannabis sativa using an in silico study. We report sixteen phytocannabinoids: cannabidiol (CBD), cannabidiolic acid (CBDA), cannabinol (CBN), cannabichromene (CBC), cannabigerol (CBG), cannabicyclol (CBL), cannabivarin (CBV), cannabidivarin (CBDV), cannabichromevarin (CBCV), cannabigerovarin (CBGV), cannabinodiol (CBDL), cannabielsoin (CBE), cannabitriol (CBT), Δ9-tetrahydrocannabinol (Δ9-THC), Δ9-tetrahydrocannabivarin (Δ9-THCV), and Δ8-tetrahydrocannabinol (Δ8-THC). All chemical structures and properties were obtained from PubChem Compound, National Center for Biotechnology Information, U.S. National Library of Medicine. Molinspiration was used for the calculation of molecular properties and bioactivity score. The parameters were molecular weight (MW), number of hydrogen acceptor (HBA), number of hydrogen donor (HBD), partition coefficient (cLogP), polar surface area (PSA) and number of rotatable bonds (NROTB). We predicted bioactivity scores for G Protein-Coupled Receptors (GPCR) ligand, ion channel modulator, kinase inhibitor, nuclear receptor ligand, protease inhibitor and enzyme inhibitor. Lipinski’s rule was used as reference to determine the drug-like properties of the phytocannabinoids. All compounds have MW<500, HBA<10, HBD<5, TPSA<140Å2 and NRTOB<10. Bioactivity score showed an active or moderately active in all compounds. -

The Use of Cannabinoids in Animals and Therapeutic Implications for Veterinary Medicine: a Review
Veterinarni Medicina, 61, 2016 (3): 111–122 Review Article doi: 10.17221/8762-VETMED The use of cannabinoids in animals and therapeutic implications for veterinary medicine: a review L. Landa1, A. Sulcova2, P. Gbelec3 1Faculty of Medicine, Masaryk University, Brno, Czech Republic 2Central European Institute of Technology, Masaryk University, Brno, Czech Republic 3Veterinary Hospital and Ambulance AA Vet, Prague, Czech Republic ABSTRACT: Cannabinoids/medical marijuana and their possible therapeutic use have received increased atten- tion in human medicine during the last years. This increased attention is also an issue for veterinarians because particularly companion animal owners now show an increased interest in the use of these compounds in veteri- nary medicine. This review sets out to comprehensively summarise well known facts concerning properties of cannabinoids, their mechanisms of action, role of cannabinoid receptors and their classification. It outlines the main pharmacological effects of cannabinoids in laboratory rodents and it also discusses examples of possible beneficial use in other animal species (ferrets, cats, dogs, monkeys) that have been reported in the scientific lit- erature. Finally, the article deals with the prospective use of cannabinoids in veterinary medicine. We have not intended to review the topic of cannabinoids in an exhaustive manner; rather, our aim was to provide both the scientific community and clinical veterinarians with a brief, concise and understandable overview of the use of cannabinoids in veterinary -

Preparative Isolation of Cannabinoids from Cannabis Sativa by Centrifugal Partition Chromatography
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/244594577 Preparative Isolation of Cannabinoids from Cannabis sativa by Centrifugal Partition Chromatography Article in Journal of Liquid Chromatography & Related Technologies · December 2004 DOI: 10.1081/JLC-200028170 CITATIONS READS 47 2,645 5 authors, including: Arno Hazekamp Robert Verpoorte Bedrocan BV Leiden University 50 PUBLICATIONS 975 CITATIONS 732 PUBLICATIONS 21,203 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: The effects of inhaled delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on pain relief and subjective effects in fibromyalgia patients View project All content following this page was uploaded by Robert Verpoorte on 20 June 2014. The user has requested enhancement of the downloaded file. Cannabis; extracting the medicine i Arno Hazekamp Cannabis; extracting the medicine Proefschrift Universiteit Leiden ISBN 978-90-9021997-4 Printed by PrintPartners Ipskamp B.V., Amsterdam, The Netherlands Paper cover: Cannabis Pur 100% (250 grams) from Grafisch Papier, The Nederlands. Photo cover: Dutch medicinal cannabis, variety “Bedrocan”. ii Cannabis; extracting the medicine Proefschrift Ter verkrijging van de graad van Doctor aan de Universiteit Leiden, op gezag van de Rector Magnificus prof. mr. P. F. van der Heijden, hoogleraar in de faculteit der Rechtsgeleerdheid, volgens besluit van het College voor Promoties te verdedigen op woensdag 5 september 2007 klokke 15.00 uur door Arno Hazekamp Geboren op 15 maart 1976 te Bilthoven iii Promotiecommissie Promotor Prof. dr. R. Verpoorte Referent Dr. C. Giroud (Institut Universitaire de Médecine Légale, Lausanne, Switzerland) Overige leden Prof. -

2 Cannabis Sativa L
CANNABIS SATIVA 29 2 Cannabis sativa L. Common Names Almindelig hamp Denmark Harilik kanep Slovenia Asa Japan Haschischpflanze Germany Bang Egypt Hash United Kingdom Bhaang India Hashas Turkey Bhaango Nepal Hashish Morocco Canamo indico Spain Hemp United Kingdom Canapa indica Italy Hennep Netherlands Canhamo Portugal Hind kinnabi Turkey Cares Nepal Huo ma cao China Chanvre cultive France Huo ma China Chanvre de l’Inde France Indian hemp United Kingdom Chanvre France Indische hennep Netherlands Chanvrier sauvage France Indischer hanf Germany Charas India Indisk hamp Sweden Churras India Kannabis Finland Da ma cao China Kannabisu Japan Da ma ren China Kerp Albania Da ma China Kinnab Turkey Dagga South Africa Konopie siewne Poland Dansk pot Denmark Konopie Poland Echter hanf Germany Konoplja Slovenia Esrar Turkey Kultur hanf Germany Gaanjaa Nepal Maconha Portugal Gajiimaa Nepal Marihana Netherlands Ganja Guyana Marihouava Greece Ganja India Marihuana Poland Grifa Spain Marihuana Bulgaria Hachis Spain Marihuana Croatia Hamp Denmark Marihuana Czech Republic Hamp Norway Marihuana Denmark Hampa Sweden Marihuana France Hampjurt Iceland Marihuana Germany Hamppu Finland Marihuana Hungary Hanf Germany Marihuana Mexico From: Medicinal Plants of the World, vol. 3: Chemical Constituents, Traditional and Modern Medicinal Uses By: I. A. Ross © Humana Press Inc., Totowa, NJ 29 30 MEDICINAL PLANTS OF THE WORLD Marihuana Russia Porkanchaa Thailand Marihuana Serbia Pot Denmark Marihuana Spain Qinnib Arabic countries Marihuana Ukraine Riesen hanf Germany Marihuana United States Seruma erva Portugal Marijuana France Taima Japan Marijuana Italy Til Arabic countries Marijuana Mexico Vrai chanvre France Marijuana Portugal Weed Guyana Marijuana Sweden Xian ma China Mashinin Japan Ye ma China Navadna konoplja Slovenia BOTANICAL DESCRIPTION types of plants are numerous varieties that Cannabis sativa is an annual herb of the differ from the main one in height, extent MORACEAE family that grows to 5 m tall.