Evolutionary Patterns of Codon Usage in the Chloroplast Gene Rbcl
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seed Plant Models
Review Tansley insight Why we need more non-seed plant models Author for correspondence: Stefan A. Rensing1,2 Stefan A. Rensing 1 2 Tel: +49 6421 28 21940 Faculty of Biology, University of Marburg, Karl-von-Frisch-Str. 8, 35043 Marburg, Germany; BIOSS Biological Signalling Studies, Email: stefan.rensing@biologie. University of Freiburg, Sch€anzlestraße 18, 79104 Freiburg, Germany uni-marburg.de Received: 30 October 2016 Accepted: 18 December 2016 Contents Summary 1 V. What do we need? 4 I. Introduction 1 VI. Conclusions 5 II. Evo-devo: inference of how plants evolved 2 Acknowledgements 5 III. We need more diversity 2 References 5 IV. Genomes are necessary, but not sufficient 3 Summary New Phytologist (2017) Out of a hundred sequenced and published land plant genomes, four are not of flowering plants. doi: 10.1111/nph.14464 This severely skewed taxonomic sampling hinders our comprehension of land plant evolution at large. Moreover, most genetically accessible model species are flowering plants as well. If we are Key words: Charophyta, evolution, fern, to gain a deeper understanding of how plants evolved and still evolve, and which of their hornwort, liverwort, moss, Streptophyta. developmental patterns are ancestral or derived, we need to study a more diverse set of plants. Here, I thus argue that we need to sequence genomes of so far neglected lineages, and that we need to develop more non-seed plant model species. revealed much, the exact branching order and evolution of the I. Introduction nonbilaterian lineages is still disputed (Lanna, 2015). Research on animals has for a long time relied on a number of The first (small) plant genome to be sequenced was of THE traditional model organisms, such as mouse, fruit fly, zebrafish or model plant, the weed Arabidopsis thaliana (c. -

Evolution of Land Plants P
Chapter 4. The evolutionary classification of land plants The evolutionary classification of land plants Land plants evolved from a group of green algae, possibly as early as 500–600 million years ago. Their closest living relatives in the algal realm are a group of freshwater algae known as stoneworts or Charophyta. According to the fossil record, the charophytes' growth form has changed little since the divergence of lineages, so we know that early land plants evolved from a branched, filamentous alga dwelling in shallow fresh water, perhaps at the edge of seasonally-desiccating pools. The biggest challenge that early land plants had to face ca. 500 million years ago was surviving in dry, non-submerged environments. Algae extract nutrients and light from the water that surrounds them. Those few algae that anchor themselves to the bottom of the waterbody do so to prevent being carried away by currents, but do not extract resources from the underlying substrate. Nutrients such as nitrogen and phosphorus, together with CO2 and sunlight, are all taken by the algae from the surrounding waters. Land plants, in contrast, must extract nutrients from the ground and capture CO2 and sunlight from the atmosphere. The first terrestrial plants were very similar to modern mosses and liverworts, in a group called Bryophytes (from Greek bryos=moss, and phyton=plants; hence “moss-like plants”). They possessed little root-like hairs called rhizoids, which collected nutrients from the ground. Like their algal ancestors, they could not withstand prolonged desiccation and restricted their life cycle to shaded, damp habitats, or, in some cases, evolved the ability to completely dry-out, putting their metabolism on hold and reviving when more water arrived, as in the modern “resurrection plants” (Selaginella). -

A Review of the Late Jurassic–Early Cretaceous Charophytes from The
A review of the Late Jurassic–Early Cretaceous charophytes from the northern Aquitaine Basin in south-west France Roch-Alexandre Benoit, Didier Néraudeau, Carles Martin-Closas To cite this version: Roch-Alexandre Benoit, Didier Néraudeau, Carles Martin-Closas. A review of the Late Jurassic– Early Cretaceous charophytes from the northern Aquitaine Basin in south-west France. Cretaceous Research, Elsevier, 2017, 79, pp.199-213. <10.1016/j.cretres.2017.07.009>. <insu-01574653> HAL Id: insu-01574653 https://hal-insu.archives-ouvertes.fr/insu-01574653 Submitted on 16 Aug 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript A review of the Late Jurassic–Early Cretaceous charophytes from the northern Aquitaine Basin in south-west France Roch-Alexandre Benoit, Didier Neraudeau, Carles Martín-Closas PII: S0195-6671(17)30121-0 DOI: 10.1016/j.cretres.2017.07.009 Reference: YCRES 3658 To appear in: Cretaceous Research Received Date: 13 March 2017 Revised Date: 5 July 2017 Accepted Date: 17 July 2017 Please cite this article as: Benoit, R.-A., Neraudeau, D., Martín-Closas, C., A review of the Late Jurassic–Early Cretaceous charophytes from the northern Aquitaine Basin in south-west France, Cretaceous Research (2017), doi: 10.1016/j.cretres.2017.07.009. -

Supplementary Information the Biodiversity and Geochemistry Of
Supplementary Information The Biodiversity and Geochemistry of Cryoconite Holes in Queen Maud Land, East Antarctica Figure S1. Principal component analysis of the bacterial OTUs. Samples cluster according to habitats. Figure S2. Principal component analysis of the eukaryotic OTUs. Samples cluster according to habitats. Figure S3. Principal component analysis of selected trace elements that cause the separation (primarily Zr, Ba and Sr). Figure S4. Partial canonical correspondence analysis of the bacterial abundances and all non-collinear environmental variables (i.e., after identification and exclusion of redundant predictor variables) and without spatial effects. Samples from Lake 3 in Utsteinen clustered with higher nitrate concentration and samples from Dubois with a higher TC abundance. Otherwise no clear trends could be observed. Table S1. Number of sequences before and after quality control for bacterial and eukaryotic sequences, respectively. 16S 18S Sample ID Before quality After quality Before quality After quality filtering filtering filtering filtering PES17_36 79285 71418 112519 112201 PES17_38 115832 111434 44238 44166 PES17_39 128336 123761 31865 31789 PES17_40 107580 104609 27128 27074 PES17_42 225182 218495 103515 103323 PES17_43 219156 213095 67378 67199 PES17_47 82531 79949 60130 59998 PES17_48 123666 120275 64459 64306 PES17_49 163446 158674 126366 126115 PES17_50 107304 104667 158362 158063 PES17_51 95033 93296 - - PES17_52 113682 110463 119486 119205 PES17_53 126238 122760 72656 72461 PES17_54 120805 117807 181725 181281 PES17_55 112134 108809 146821 146408 PES17_56 193142 187986 154063 153724 PES17_59 226518 220298 32560 32444 PES17_60 186567 182136 213031 212325 PES17_61 143702 140104 155784 155222 PES17_62 104661 102291 - - PES17_63 114068 111261 101205 100998 PES17_64 101054 98423 70930 70674 PES17_65 117504 113810 192746 192282 Total 3107426 3015821 2236967 2231258 Table S2. -

The Charophytes (Charophyta) Locality in the Milkha Stream, Lower Jordan, Israel
Natural Resources and Conservation 3(2): 19-30, 2015 http://www.hrpub.org DOI: 10.13189/nrc.2015.030201 The Charophytes (Charophyta) Locality in the Milkha Stream, Lower Jordan, Israel Sophia Barinova1,*, Roman Romanov2 1Institute of Evolution, University of Haifa, Israel 2Central Siberian Botanical Garden of the Siberian Branch of the Russian Academy of Sciences, Russia Copyright © 2015 by authors, all rights reserved. Authors agree that this article remains permanently open access under the terms of the Creative Commons Attribution License 4.0 International License Abstract First study of new locality the Milkha Stream, revealed 14 charophyte species (16 with ifraspecific variety) the Lower Jordan River tributary, with charophyte algae, in that known for Israel [3,4] from references and our studies. semi-arid region of Israel has been implemented for Last year research in Israel and Eastern Mediterranean let us revealing of algal diversity and ecological assessment of the too includes few new localities, algal diversity of which has water object environment by bio-indication methods. been never studied before [5-10]. Altogether forty seven species from five taxonomic The charophytes prefer alkaline water environment which Divisions of algae and cyanobacteria including one of them forms on the carbonates that are very distributed in studied macro-algae Chara gymnophylla A. Braun were revealed in region. This alkaline freshwater environment can be assessed the Milkha stream. Chara was found in growth in the lower as perspective for find new, unstudied aquatic objects in part of studied stream but away from community in followed which can be identified charophyte algae. The most years. -

Conifers Exhibit a Characteristic Inactivation of Auxin to Maintain Tissue
bioRxiv preprint doi: https://doi.org/10.1101/789420; this version posted October 1, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Title Conifers exhibit a characteristic inactivation of auxin to maintain tissue homeostasis Authors Federica Brunoni1,2,a, Silvio Collani1, Rubén Casanova-Saéz2, Jan Šimura2, Michal Karady2,a, Markus Schmid1, Karin Ljung2,b and Catherine Bellini1,3,b 1 Umeå Plant Science Centre, Dept of Plant Physiology, Umeå University (Umu), Umeå, Sweden 2 Umeå Plant Science Centre, Dept of Forest Genetics and Plant Physiology, Swedish University of Agricultural Sciences (SLU), Umeå, Sweden 3 Institut Jean-Pierre Bourgin, UMR1318 INRA-AgroParisTech Versailles, France a Present address: Laboratory of Growth Regulators, Faculty of Science, Palacký University & Institute of Experimental Botany, The Czech Academy of Sciences, Šlechtitelů 27, CZ- 78371, Olomouc, Czech Republic b to whom correspondence may be addressed. Email: [email protected] or [email protected] or [email protected] Summary • Dynamic regulation of the levels of the natural auxin, indol-3-acetic acid (IAA), is essential to coordinate most of the physiological and developmental processes and responses to environmental changes. Oxidation of IAA is a major pathway to control auxin concentrations in Arabidopsis and, along with IAA conjugation, to respond to perturbation of IAA homeostasis. However, these regulatory mechanisms are still poorly investigated in conifers. To reduce this gap of knowledge, we investigated the different contribution of the IAA inactivation pathways in conifers. • Mass spectrometry-based quantification of IAA metabolites under steady state conditions and after perturbation was investigated to evaluate IAA homeostasis in bioRxiv preprint doi: https://doi.org/10.1101/789420; this version posted October 1, 2019. -
2021-1 RL Stats Table1a.Pdf
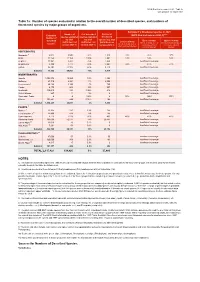
IUCN Red List version 2021-1: Table 1a Last updated: 25 March 2021 Table 1a: Number of species evaluated in relation to the overall number of described species, and numbers of threatened species by major groups of organisms. Estimated % threatened species in 2021 Number of % of described Number of 2,3,4 Estimated (IUCN Red List version 2021-1) species evaluated species evaluated threatened Number of 2 by 2021 by 2021 species by 2021 Best estimate Upper estimate described Lower estimate 1 (IUCN Red List (IUCN Red List (IUCN Red List (threatened spp. as % of (threatened and DD spp. as species (threatened spp. as % of version 2021-1) version 2021-1) extant data sufficient % of extant evaluated version 2021-1) extant evaluated species) evaluated species) species) VERTEBRATES Mammals 5 6,513 5,940 91% 1,323 23% 26% 37% Birds 11,158 11,158 100% 1,481 13% 14% 14% Reptiles 11,341 8,492 75% 1,458 Insufficient coverage Amphibians 8,309 7,212 87% 2,442 34% 41% 51% Fishes 35,797 22,005 61% 3,210 Insufficient coverage Subtotal 73,118 54,807 75% 9,914 INVERTEBRATES Insects 1,053,578 10,865 1.0% 1,926 Insufficient coverage Molluscs 81,719 8,881 11% 2,305 Insufficient coverage Crustaceans6 80,122 3,189 4% 743 Insufficient coverage Corals 2,175 864 40% 237 Insufficient coverage Arachnids 110,615 393 0.36% 218 Insufficient coverage Velvet Worms 227 11 5% 9 Insufficient coverage Horseshoe Crabs 4 4 100% 2 50% 100% 100% Others 151,801 844 0.56% 148 Insufficient coverage Subtotal 1,480,241 25,051 2% 5,588 PLANTS 7 Mosses 8 21,925 282 1.3% 165 Insufficient coverage Ferns and Allies 9 11,800 678 6% 266 Insufficient coverage Gymnosperms 1,113 1,016 91% 403 40% 41% 42% Flowering Plants 369,000 52,077 14% 20,883 Insufficient coverage Green Algae 10 11,616 16 0.1% 0 Insufficient coverage Red Algae 10 7,291 58 0.8% 9 Insufficient coverage Subtotal 422,745 54,127 13% 21,726 FUNGI & PROTISTS 11 Lichens 17,000 57 0.3% 50 Insufficient coverage Mushrooms, etc. -

TAS3 Mir390-Dependent Loci in Non-Vascular Land Plants: Towards a Comprehensive Reconstruction of the Gene Evolutionary History
TAS3 miR390-dependent loci in non-vascular land plants: towards a comprehensive reconstruction of the gene evolutionary history Sergey Y. Morozov1, Irina A. Milyutina1, Tatiana N. Erokhina2, Liudmila V. Ozerova3, Alexey V. Troitsky1 and Andrey G. Solovyev1,4 1 Belozersky Institute of Physico-Chemical Biology, Moscow State University, Moscow, Russia 2 Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Science, Moscow, Russia 3 Tsitsin Main Botanical Garden, Russian Academy of Science, Moscow, Russia 4 Institute of Molecular Medicine, Sechenov First Moscow State Medical University, Moscow, Russia ABSTRACT Trans-acting small interfering RNAs (ta-siRNAs) are transcribed from protein non- coding genomic TAS loci and belong to a plant-specific class of endogenous small RNAs. These siRNAs have been found to regulate gene expression in most taxa including seed plants, gymnosperms, ferns and mosses. In this study, bioinformatic and experimental PCR-based approaches were used as tools to analyze TAS3 and TAS6 loci in transcriptomes and genomic DNAs from representatives of evolutionary distant non-vascular plant taxa such as Bryophyta, Marchantiophyta and Anthocero- tophyta. We revealed previously undiscovered TAS3 loci in plant classes Sphagnopsida and Anthocerotopsida, as well as TAS6 loci in Bryophyta classes Tetraphidiopsida, Polytrichopsida, Andreaeopsida and Takakiopsida. These data further unveil the evolutionary pathway of the miR390-dependent TAS3 loci in land plants. We also identified charophyte alga sequences coding for SUPPRESSOR OF GENE SILENCING 3 (SGS3), which is required for generation of ta-siRNAs in plants, and hypothesized that the appearance of TAS3-related sequences could take place at a very early step in Submitted 19 February 2018 evolutionary transition from charophyte algae to an earliest common ancestor of land Accepted 28 March 2018 plants. -

Some Charophyta (Charales) from Coastal Temporary Ponds in Velipoja Area (North Albania)
Journal of Environmental Science and Engineering B 5 (2016) 69-77 doi:10.17265/2162-5263/2016.02.002 D DAVID PUBLISHING Some Charophyta (Charales) from Coastal Temporary Ponds in Velipoja Area (North Albania) Vilza Zeneli1 and Lefter Kashta2 1. Department of Biology, Faculty of Natural Sciences, The University of Tirana, Tirana 1001, Albania 2. Research Center of Flora and Fauna, Faculty of Natural Sciences, The University of Tirana, Tirana 1001, Albania Abstract: Charophytes or stoneworts constitute a group of macrophytes that occur mostly in fresh-water environments but can also be found in brackish waters. Knowledge about stoneworts in Albania is still scarce and incomplete. According to published data on charoflora of Albania, there are 24 species and four genera known from different freshwater habitats. The present work is based on plant material sampled from 5 slightly brackish-water temporary ponds in the coastal area of Velipoja (north Albania). During spring and summer of 2013-2015, field surveys were carried out with the main purpose of filling knowledge gaps concerning brackish water charophytes. Altogether seven species were identified: four typical of brackish water habitat (Chara baltica, Chara canescens, Chara galioides and Chara connivens) and three of broader tolerance (Chara aspera, Chara vulgaris and Tolypella glomerata). The first three species, which considered as the rarest and most threatened on the Balkans were found for the first time in Albania. Key words: Charophyta, brackish water, Albania, Velipoja area, temporary ponds. 1. Introduction (one species) and Tolypella (one species) were reported for Albania from different freshwater habitats Charophytes or stoneworts are a group of like lakes, rivers, ponds, etc. -

Insights Into the Red Algae and Eukaryotic Evolution From
Insights into the red algae and eukaryotic evolution PNAS PLUS from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta) Susan H. Brawleya,1, Nicolas A. Blouina,b, Elizabeth Ficko-Bleanc, Glen L. Wheelerd, Martin Lohre, Holly V. Goodsonf, Jerry W. Jenkinsg,h, Crysten E. Blaby-Haasi, Katherine E. Helliwelld,j, Cheong Xin Chank,l, Tara N. Marriagem, Debashish Bhattacharyan, Anita S. Kleino, Yacine Badisp, Juliet Brodieq, Yuanyu Caoo,2, Jonas Collénc, Simon M. Dittamic, Claire M. M. Gachonp, Beverley R. Greenr, Steven J. Karpowiczs, Jay W. Kimt, Ulrich Johan Kudahlj, Senjie Linu, Gurvan Michelc, Maria Mittagv, Bradley J. S. C. Olsonm, Jasmyn L. Pangilinanh, Yi Pengh, Huan Qiun, Shengqiang Shuh, John T. Singerw, Alison G. Smithj, Brittany N. Sprecheru, Volker Wagnerv, Wenfei Wangx, Zhi-Yong Wangy, Juying Yanh, Charles Yarishz, Simone Zäuner-Riekaa, Yunyun Zhuangu,3, Yong Zouv, Erika A. Lindquisth, Jane Grimwoodg,h, Kerrie W. Barryh, Daniel S. Rokhsarh, Jeremy Schmutzg,h, John W. Stillerbb, Arthur R. Grossmany, and Simon E. Prochnikh aSchool of Marine Sciences, University of Maine, Orono, ME 04469; bDepartment of Molecular Biology, University of Wyoming, Laramie, WY 82071; cSorbonne Universités, Université Pierre and Marie Curie Paris 06, CNRS, UMR 8227, Integrative Biology of Marine Models, Station Biologique de Roscoff, CS 90074, 29688 Roscoff, France; dMarine Biological Association of the United Kingdom, Plymouth, PL1 2PB, United Kingdom; eInstitut für Molekulare Physiologie, Pflanzenbiochemie, Johannes Gutenberg-Universität Mainz, -

Evolutionary Transitions Between States of Structural and Developmental Characters Among the Algal Charophyta (Viridiplantae)
ABSTRACT Title of Thesis: EVOLUTIONARY TRANSITIONS BETWEEN STATES OF STRUCTURAL AND DEVELOPMENTAL CHARACTERS AMONG THE ALGAL CHAROPHYTA (VIRIDIPLANTAE). Jeffrey David Lewandowski, Master of Science, 2004 Thesis Directed By: Associate Profe ssor, Charles F. Delwiche, Cell Biology and Molecular Genetics The Charophyta comprise green plant representatives ranging from familiar complex -bodied land plants to subtle and simple forms of green algae, presumably the closest phylogenetic relatives of land plants. This biological lineage provides a unique opportunity to investigate evolutionary transition series that likely facilitated once -aquatic green plants to colonize and diversify in terrestrial environments. A literature review summarizes fu ndamental structural and developmental transitions observed among the major lineages of algal Charophyta. A phylogenetic framework independent of morphological and ontological data is necessary for testing hypotheses about the evolution of structure and development. Thus, to further elucidate the branching order of the algal Charophyta, new DNA sequence data are used to test conflicting hypotheses regarding the phylogenetic placement of several enigmatic taxa, including the algal charophyte genera Mesosti gma , Chlorokybus, Coleochaete , and Chaetosphaeridium. Additionally, technical notes on developing RNA methods for use in studying algal Charophyta are included. EVOLUTIONARY TRANSITIONS BETWEEN STATES OF STRUCTURAL AND DEVELOPMENTAL CHARACTERS AMONG THE ALGAL CHAROPHYTA (VIRIDIPLANTAE). By Jeffrey David Lewandowski Thesis submitted to the Faculty of the Graduate School of the University of Maryland, College Park, in partial fulfillment of the requirements for the degree of Master of Science 2004 Advisory Committee: Associate Professor Charles F. Delwiche, Chair Professor Todd J. Cooke Assistant Professor Eric S. Haag © Copyright by Jeffrey D. Lewandowski 2004 Preface The following are examples from several of the works that have provided philosophical inspiration for this document. -

Some Preliminary Experiments on Physiology of Charophyta.1 by V
Some preliminary Experiments on Physiology of Charophyta.1 By V. V ouk and F. Benzinger. The Charophyta have been frequently the object of study of the appearance of streaming of protoplasm, yet the fundamental questions regarding their physiology remained almost wholly untouched. The Charophyta, biologically, represent a fairly devel oped type among the Thallophyta. Regarding nutrition they remind us of higher plants because of the presence of rhizoids. According to Bierb e r g ’s investigations the rhizoids not only perform the function of fixations, but also the main function of nutrition. The nutritive materials are — to our knowledge — not yet analysed. Occasional observations only have been made so far, namely: that the growth of Charophyta varies in different soils (M ig u la, E rn s t). We know just as little about the external factors of the growth of the Charophyta such as light and temperature. Only lately Karl Fug investigated the activity of light. From observation in nature we know the great influence1 of light upon the growth and fructification of Charophyta. The few investigations on Charophyta led to some preliminary fundamental experiments with Charophyta. The following questions were put: 1. How will Charophyta absorbe nutritive materials? Are the rhizoids the only organs of absorbtion or is the surface of thallus performing the same function? 2. What is the growth of Charophyta in differing soils? 3. What is the relation of the Charophyta to the light factor? In what way does light influence the growth of the vege tative organs and their propagation? 4. What is1 the/ relation of the Charophyta to mineral nutrition? Which mineral nutritive material is indispensable to Charo phyta? 1 This communication contains iu condensed form part of the results of experimental work on Charophyta proposed by me and carried' out by Mr.