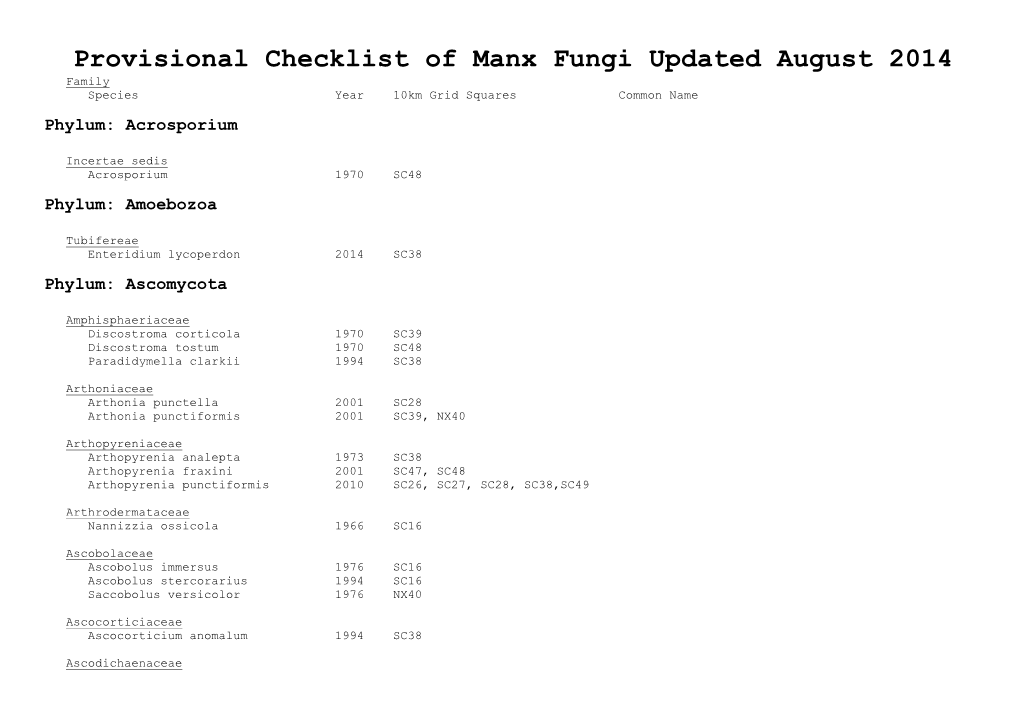
Provisional Checklist of Manx Fungi Updated August 2014 Family Species Year 10Km Grid Squares Common Name
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Agaricales, Basidiomycota) Occurring in Punjab, India
Current Research in Environmental & Applied Mycology 5 (3): 213–247(2015) ISSN 2229-2225 www.creamjournal.org Article CREAM Copyright © 2015 Online Edition Doi 10.5943/cream/5/3/6 Ecology, Distribution Perspective, Economic Utility and Conservation of Coprophilous Agarics (Agaricales, Basidiomycota) Occurring in Punjab, India Amandeep K1*, Atri NS2 and Munruchi K2 1Desh Bhagat College of Education, Bardwal–Dhuri–148024, Punjab, India. 2Department of Botany, Punjabi University, Patiala–147002, Punjab, India. Amandeep K, Atri NS, Munruchi K 2015 – Ecology, Distribution Perspective, Economic Utility and Conservation of Coprophilous Agarics (Agaricales, Basidiomycota) Occurring in Punjab, India. Current Research in Environmental & Applied Mycology 5(3), 213–247, Doi 10.5943/cream/5/3/6 Abstract This paper includes the results of eco-taxonomic studies of coprophilous mushrooms in Punjab, India. The information is based on the survey to dung localities of the state during the various years from 2007-2011. A total number of 172 collections have been observed, growing as saprobes on dung of various domesticated and wild herbivorous animals in pastures, open areas, zoological parks, and on dung heaps along roadsides or along village ponds, etc. High coprophilous mushrooms’ diversity has been established and a number of rare and sensitive species recorded with the present study. The observed collections belong to 95 species spread over 20 genera and 07 families of the order Agaricales. The present paper discusses the distribution of these mushrooms in Punjab among different seasons, regions, habitats, and growing habits along with their economic utility, habitat management and conservation. This is the first attempt in which various dung localities of the state has been explored systematically to ascertain the diversity, seasonal availability, distribution and ecology of coprophilous mushrooms. -

Diversity, Nutritional Composition and Medicinal Potential of Indian Mushrooms: a Review
Vol. 13(4), pp. 523-545, 22 January, 2014 DOI: 10.5897/AJB2013.13446 ISSN 1684-5315 ©2014 Academic Journals African Journal of Biotechnology http://www.academicjournals.org/AJB Review Diversity, nutritional composition and medicinal potential of Indian mushrooms: A review Hrudayanath Thatoi* and Sameer Kumar Singdevsachan Department of Biotechnology, College of Engineering and Technology, Biju Patnaik University of Technology, Bhubaneswar-751003, Odisha, India. Accepted 2 January, 2014 Mushrooms are the higher fungi which have long been used for food and medicinal purposes. They have rich nutritional value with high protein content (up to 44.93%), vitamins, minerals, fibers, trace elements and low calories and lack cholesterol. There are 14,000 known species of mushrooms of which 2,000 are safe for human consumption and about 650 of these possess medicinal properties. Among the total known mushrooms, approximately 850 species are recorded from India. Many of them have been used in food and folk medicine for thousands of years. Mushrooms are also sources of bioactive substances including antibacterial, antifungal, antiviral, antioxidant, antiinflammatory, anticancer, antitumour, anti-HIV and antidiabetic activities. Nutriceuticals and medicinal mushrooms have been used in human health development in India as food, medicine, minerals among others. The present review aims to update the current status of mushrooms diversity in India with their nutritional and medicinal potential as well as ethnomedicinal uses for different future prospects in pharmaceutical application. Key words: Mushroom diversity, nutritional value, therapeutic potential, bioactive compound. INTRODUCTION Mushroom is a general term used mainly for the fruiting unexamined mushrooms will be only 5%, implies that body of macrofungi (Ascomycota and Basidiomycota) there are 7,000 yet undiscovered species, which if and represents only a short reproductive stage in their life discovered will be provided with the possible benefit to cycle (Das, 2010). -

Thyronectria Revisited and Re-Instated
Persoonia 33, 2014: 182–211 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE http://dx.doi.org/10.3767/003158514X685211 Persistent hamathecial threads in the Nectriaceae, Hypocreales: Thyronectria revisited and re-instated W.M. Jaklitsch1, H. Voglmayr1,2 Key words Abstract Based on type studies and freshly collected material we here re-instate the genus Thyronectria (Nec- triaceae, Hypocreales). Species of this genus were recently for the most part classified in the genera Pleonectria act (Nectriaceae) or Mattirolia (Thyridiaceae), because Thyronectria and other genera had been identified as members Ascomycota of the Thyridiaceae due to the presence of paraphyses. Molecular phylogenies based on several markers (act, ITS, Hypocreales LSU rDNA, rpb1, rpb2, tef1, tub) revealed that the Nectriaceae contain members whose ascomata are characterised Mattirolia by long, more or less persistent, apical paraphyses. All of these belong to a single genus, Thyronectria, which thus Nectriaceae has representatives with hyaline, rosy, green or even dark brown and sometimes distoseptate ascospores. The new species type species of Thyronectria, T. rhodochlora, syn. T. patavina, syn. T. pyrrhochlora is re-described and illustrated. Pleonectria Within the Nectriaceae persistent, apical paraphyses are common in Thyronectria and rarely also occur in Nectria. pyrenomycetes The genus Mattirolia is revised and merged with Thyronectria and also Thyronectroidea is regarded as a synonym rpb1 of Thyronectria. The three new species T. asturiensis, T. caudata and T. obscura are added to the genus. Species rpb2 recently described in Pleonectria as well as some species of Mattirolia are combined in the genus, and a key to tef1 Thyronectria is provided. Five species are epitypified. -

Pt Reyes Species As of 12-1-2017 Abortiporus Biennis Agaricus
Pt Reyes Species as of 12-1-2017 Abortiporus biennis Agaricus augustus Agaricus bernardii Agaricus californicus Agaricus campestris Agaricus cupreobrunneus Agaricus diminutivus Agaricus hondensis Agaricus lilaceps Agaricus praeclaresquamosus Agaricus rutilescens Agaricus silvicola Agaricus subrutilescens Agaricus xanthodermus Agrocybe pediades Agrocybe praecox Alboleptonia sericella Aleuria aurantia Alnicola sp. Amanita aprica Amanita augusta Amanita breckonii Amanita calyptratoides Amanita constricta Amanita gemmata Amanita gemmata var. exannulata Amanita calyptraderma Amanita calyptraderma (white form) Amanita magniverrucata Amanita muscaria Amanita novinupta Amanita ocreata Amanita pachycolea Amanita pantherina Amanita phalloides Amanita porphyria Amanita protecta Amanita velosa Amanita smithiana Amaurodon sp. nova Amphinema byssoides gr. Annulohypoxylon thouarsianum Anthrocobia melaloma Antrodia heteromorpha Aphanobasidium pseudotsugae Armillaria gallica Armillaria mellea Armillaria nabsnona Arrhenia epichysium Pt Reyes Species as of 12-1-2017 Arrhenia retiruga Ascobolus sp. Ascocoryne sarcoides Astraeus hygrometricus Auricularia auricula Auriscalpium vulgare Baeospora myosura Balsamia cf. magnata Bisporella citrina Bjerkandera adusta Boidinia propinqua Bolbitius vitellinus Suillellus (Boletus) amygdalinus Rubroboleus (Boletus) eastwoodiae Boletus edulis Boletus fibrillosus Botryobasidium longisporum Botryobasidium sp. Botryobasidium vagum Bovista dermoxantha Bovista pila Bovista plumbea Bulgaria inquinans Byssocorticium californicum -

The Fungi of Slapton Ley National Nature Reserve and Environs
THE FUNGI OF SLAPTON LEY NATIONAL NATURE RESERVE AND ENVIRONS APRIL 2019 Image © Visit South Devon ASCOMYCOTA Order Family Name Abrothallales Abrothallaceae Abrothallus microspermus CY (IMI 164972 p.p., 296950), DM (IMI 279667, 279668, 362458), N4 (IMI 251260), Wood (IMI 400386), on thalli of Parmelia caperata and P. perlata. Mainly as the anamorph <it Abrothallus parmeliarum C, CY (IMI 164972), DM (IMI 159809, 159865), F1 (IMI 159892), 2, G2, H, I1 (IMI 188770), J2, N4 (IMI 166730), SV, on thalli of Parmelia carporrhizans, P Abrothallus parmotrematis DM, on Parmelia perlata, 1990, D.L. Hawksworth (IMI 400397, as Vouauxiomyces sp.) Abrothallus suecicus DM (IMI 194098); on apothecia of Ramalina fustigiata with st. conid. Phoma ranalinae Nordin; rare. (L2) Abrothallus usneae (as A. parmeliarum p.p.; L2) Acarosporales Acarosporaceae Acarospora fuscata H, on siliceous slabs (L1); CH, 1996, T. Chester. Polysporina simplex CH, 1996, T. Chester. Sarcogyne regularis CH, 1996, T. Chester; N4, on concrete posts; very rare (L1). Trimmatothelopsis B (IMI 152818), on granite memorial (L1) [EXTINCT] smaragdula Acrospermales Acrospermaceae Acrospermum compressum DM (IMI 194111), I1, S (IMI 18286a), on dead Urtica stems (L2); CY, on Urtica dioica stem, 1995, JLT. Acrospermum graminum I1, on Phragmites debris, 1990, M. Marsden (K). Amphisphaeriales Amphisphaeriaceae Beltraniella pirozynskii D1 (IMI 362071a), on Quercus ilex. Ceratosporium fuscescens I1 (IMI 188771c); J1 (IMI 362085), on dead Ulex stems. (L2) Ceriophora palustris F2 (IMI 186857); on dead Carex puniculata leaves. (L2) Lepteutypa cupressi SV (IMI 184280); on dying Thuja leaves. (L2) Monographella cucumerina (IMI 362759), on Myriophyllum spicatum; DM (IMI 192452); isol. ex vole dung. (L2); (IMI 360147, 360148, 361543, 361544, 361546). -

Preliminary Classification of Leotiomycetes
Mycosphere 10(1): 310–489 (2019) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/10/1/7 Preliminary classification of Leotiomycetes Ekanayaka AH1,2, Hyde KD1,2, Gentekaki E2,3, McKenzie EHC4, Zhao Q1,*, Bulgakov TS5, Camporesi E6,7 1Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China 2Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai, 57100, Thailand 3School of Science, Mae Fah Luang University, Chiang Rai, 57100, Thailand 4Landcare Research Manaaki Whenua, Private Bag 92170, Auckland, New Zealand 5Russian Research Institute of Floriculture and Subtropical Crops, 2/28 Yana Fabritsiusa Street, Sochi 354002, Krasnodar region, Russia 6A.M.B. Gruppo Micologico Forlivese “Antonio Cicognani”, Via Roma 18, Forlì, Italy. 7A.M.B. Circolo Micologico “Giovanni Carini”, C.P. 314 Brescia, Italy. Ekanayaka AH, Hyde KD, Gentekaki E, McKenzie EHC, Zhao Q, Bulgakov TS, Camporesi E 2019 – Preliminary classification of Leotiomycetes. Mycosphere 10(1), 310–489, Doi 10.5943/mycosphere/10/1/7 Abstract Leotiomycetes is regarded as the inoperculate class of discomycetes within the phylum Ascomycota. Taxa are mainly characterized by asci with a simple pore blueing in Melzer’s reagent, although some taxa have lost this character. The monophyly of this class has been verified in several recent molecular studies. However, circumscription of the orders, families and generic level delimitation are still unsettled. This paper provides a modified backbone tree for the class Leotiomycetes based on phylogenetic analysis of combined ITS, LSU, SSU, TEF, and RPB2 loci. In the phylogenetic analysis, Leotiomycetes separates into 19 clades, which can be recognized as orders and order-level clades. -

Redalyc.Assessment of Non-Cultured Aquatic Fungal Diversity from Differenthabitats in Mexico
Revista Mexicana de Biodiversidad ISSN: 1870-3453 [email protected] Universidad Nacional Autónoma de México México Valderrama, Brenda; Paredes-Valdez, Guadalupe; Rodríguez, Rocío; Romero-Guido, Cynthia; Martínez, Fernando; Martínez-Romero, Julio; Guerrero-Galván, Saúl; Mendoza- Herrera, Alberto; Folch-Mallol, Jorge Luis Assessment of non-cultured aquatic fungal diversity from differenthabitats in Mexico Revista Mexicana de Biodiversidad, vol. 87, núm. 1, marzo, 2016, pp. 18-28 Universidad Nacional Autónoma de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=42546734003 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Available online at www.sciencedirect.com Revista Mexicana de Biodiversidad Revista Mexicana de Biodiversidad 87 (2016) 18–28 www.ib.unam.mx/revista/ Taxonomy and systematics Assessment of non-cultured aquatic fungal diversity from different habitats in Mexico Estimación de la diversidad de hongos acuáticos no-cultivables de diferentes hábitats en México a a b b Brenda Valderrama , Guadalupe Paredes-Valdez , Rocío Rodríguez , Cynthia Romero-Guido , b c d Fernando Martínez , Julio Martínez-Romero , Saúl Guerrero-Galván , e b,∗ Alberto Mendoza-Herrera , Jorge Luis Folch-Mallol a Instituto de Biotecnología, Universidad Nacional Autónoma de México, Avenida Universidad 2001, Col. Chamilpa, 62210 Cuernavaca, Morelos, Mexico b Centro de Investigación en Biotecnología, Universidad Autónoma del Estado de Morelos, Avenida Universidad 1001, Col. Chamilpa, 62209 Cuernavaca, Morelos, Mexico c Centro de Ciencias Genómicas, Universidad Nacional Autónoma de México, Avenida Universidad s/n, Col. -

9B Taxonomy to Genus
Fungus and Lichen Genera in the NEMF Database Taxonomic hierarchy: phyllum > class (-etes) > order (-ales) > family (-ceae) > genus. Total number of genera in the database: 526 Anamorphic fungi (see p. 4), which are disseminated by propagules not formed from cells where meiosis has occurred, are presently not grouped by class, order, etc. Most propagules can be referred to as "conidia," but some are derived from unspecialized vegetative mycelium. A significant number are correlated with fungal states that produce spores derived from cells where meiosis has, or is assumed to have, occurred. These are, where known, members of the ascomycetes or basidiomycetes. However, in many cases, they are still undescribed, unrecognized or poorly known. (Explanation paraphrased from "Dictionary of the Fungi, 9th Edition.") Principal authority for this taxonomy is the Dictionary of the Fungi and its online database, www.indexfungorum.org. For lichens, see Lecanoromycetes on p. 3. Basidiomycota Aegerita Poria Macrolepiota Grandinia Poronidulus Melanophyllum Agaricomycetes Hyphoderma Postia Amanitaceae Cantharellales Meripilaceae Pycnoporellus Amanita Cantharellaceae Abortiporus Skeletocutis Bolbitiaceae Cantharellus Antrodia Trichaptum Agrocybe Craterellus Grifola Tyromyces Bolbitius Clavulinaceae Meripilus Sistotremataceae Conocybe Clavulina Physisporinus Trechispora Hebeloma Hydnaceae Meruliaceae Sparassidaceae Panaeolina Hydnum Climacodon Sparassis Clavariaceae Polyporales Gloeoporus Steccherinaceae Clavaria Albatrellaceae Hyphodermopsis Antrodiella -

Ascomicetos Asociados a Angiospermas En El Bosque Mesófilo De Montaña Del Centro De Veracruz, México
Artículo de investigación Ascomicetos asociados a angiospermas en el bosque mesófilo de montaña del centro de Veracruz, México Ascomycetes associated to angiosperms in the cloud forest of central Veracruz, Mexico Rosario Medel-Ortiz1,4 , Francisco G. Lorea-Hernández2 , Yajaira Baeza Guzmán3 , Elvia N. Palestina-Villa1 , M. Emilia Belingheri Lagunes1 Resumen: Antecedentes y Objetivos: Aún se conoce poco de los hongos ascomicetos asociados a angiospermas que habitan en el bosque mesófilo de montaña en México, por lo que el objetivo del presente trabajo es incrementar el conocimiento de estos hongos que habitan en ese tipo de vegetación en el estado de Veracruz, México. Métodos: Se realizaron muestreos oportunistas en cinco localidades con fragmentos de bosque mesófilo de montaña en los municipios Coatepec, San Andrés Tlalnelhuayocan y Xalapa. Los especímenes se estudiaron macro y microscópicamente siguiendo las técnicas rutinarias para ascomicetos, utilizando literatura especializada para su identificación. Siempre que fue posible se identificó el hospedero sobre el cual estaban creciendo. Resultados clave: Se encontraron 13 especies de ascomicetos pertenecientes a los órdenes Diaporthales (familia Gnomoniaceae), Helotiales (Der- mateaceae, Helotiaceae), Hypocreales (Bionectriaceae, Niessliaceae), Orbiliales (Orbiliaceae) y Pleosporales (Massarinaceae, Montagnulaceae). De las especies estudiadas siete son nuevos registros para México, dos para Veracruz, una pertenece a una especie no descrita y tres son especies pre- viamente citadas para el país, pero se añaden nuevos datos descriptivos. Hydropisphaera suffulta se encontró creciendo de manera natural con su anamorfo Acremonium sp. Además, se cita un caso de micoparasitismo entre Letendraea helminthicola y Helminthosporium velutinum. Los hongos se encontraron creciendo en individuos de las familias Altingiaceae (Liquidambar styraciflua), Boraginaceae (Varronia sp.), Fabaceae (Inga inicuil), Fagaceae (Quercus sp.), Piperaceae (Piper spp.) y cuatro sobre madera de origen desconocido. -

Chemical Elements in Ascomycetes and Basidiomycetes
Chemical elements in Ascomycetes and Basidiomycetes The reference mushrooms as instruments for investigating bioindication and biodiversity Roberto Cenci, Luigi Cocchi, Orlando Petrini, Fabrizio Sena, Carmine Siniscalco, Luciano Vescovi Editors: R. M. Cenci and F. Sena EUR 24415 EN 2011 1 The mission of the JRC-IES is to provide scientific-technical support to the European Union’s policies for the protection and sustainable development of the European and global environment. European Commission Joint Research Centre Institute for Environment and Sustainability Via E.Fermi, 2749 I-21027 Ispra (VA) Italy Legal Notice Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of this publication. Europe Direct is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server http://europa.eu/ JRC Catalogue number: LB-NA-24415-EN-C Editors: R. M. Cenci and F. Sena JRC65050 EUR 24415 EN ISBN 978-92-79-20395-4 ISSN 1018-5593 doi:10.2788/22228 Luxembourg: Publications Office of the European Union Translation: Dr. Luca Umidi © European Union, 2011 Reproduction is authorised provided the source is acknowledged Printed in Italy 2 Attached to this document is a CD containing: • A PDF copy of this document • Information regarding the soil and mushroom sampling site locations • Analytical data (ca, 300,000) on total samples of soils and mushrooms analysed (ca, 10,000) • The descriptive statistics for all genera and species analysed • Maps showing the distribution of concentrations of inorganic elements in mushrooms • Maps showing the distribution of concentrations of inorganic elements in soils 3 Contact information: Address: Roberto M. -

80130Dimou7-107Weblist Changed
Posted June, 2008. Summary published in Mycotaxon 104: 39–42. 2008. Mycodiversity studies in selected ecosystems of Greece: IV. Macrofungi from Abies cephalonica forests and other intermixed tree species (Oxya Mt., central Greece) 1 2 1 D.M. DIMOU *, G.I. ZERVAKIS & E. POLEMIS * [email protected] 1Agricultural University of Athens, Lab. of General & Agricultural Microbiology, Iera Odos 75, GR-11855 Athens, Greece 2 [email protected] National Agricultural Research Foundation, Institute of Environmental Biotechnology, Lakonikis 87, GR-24100 Kalamata, Greece Abstract — In the course of a nine-year inventory in Mt. Oxya (central Greece) fir forests, a total of 358 taxa of macromycetes, belonging in 149 genera, have been recorded. Ninety eight taxa constitute new records, and five of them are first reports for the respective genera (Athelopsis, Crustoderma, Lentaria, Protodontia, Urnula). One hundred and one records for habitat/host/substrate are new for Greece, while some of these associations are reported for the first time in literature. Key words — biodiversity, macromycetes, fir, Mediterranean region, mushrooms Introduction The mycobiota of Greece was until recently poorly investigated since very few mycologists were active in the fields of fungal biodiversity, taxonomy and systematic. Until the end of ’90s, less than 1.000 species of macromycetes occurring in Greece had been reported by Greek and foreign researchers. Practically no collaboration existed between the scientific community and the rather few amateurs, who were active in this domain, and thus useful information that could be accumulated remained unexploited. Until then, published data were fragmentary in spatial, temporal and ecological terms. The authors introduced a different concept in their methodology, which was based on a long-term investigation of selected ecosystems and monitoring-inventorying of macrofungi throughout the year and for a period of usually 5-8 years. -

Phd. Thesis Sana Jabeen.Pdf
ECTOMYCORRHIZAL FUNGAL COMMUNITIES ASSOCIATED WITH HIMALAYAN CEDAR FROM PAKISTAN A dissertation submitted to the University of the Punjab in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in BOTANY by SANA JABEEN DEPARTMENT OF BOTANY UNIVERSITY OF THE PUNJAB LAHORE, PAKISTAN JUNE 2016 TABLE OF CONTENTS CONTENTS PAGE NO. Summary i Dedication iii Acknowledgements iv CHAPTER 1 Introduction 1 CHAPTER 2 Literature review 5 Aims and objectives 11 CHAPTER 3 Materials and methods 12 3.1. Sampling site description 12 3.2. Sampling strategy 14 3.3. Sampling of sporocarps 14 3.4. Sampling and preservation of fruit bodies 14 3.5. Morphological studies of fruit bodies 14 3.6. Sampling of morphotypes 15 3.7. Soil sampling and analysis 15 3.8. Cleaning, morphotyping and storage of ectomycorrhizae 15 3.9. Morphological studies of ectomycorrhizae 16 3.10. Molecular studies 16 3.10.1. DNA extraction 16 3.10.2. Polymerase chain reaction (PCR) 17 3.10.3. Sequence assembly and data mining 18 3.10.4. Multiple alignments and phylogenetic analysis 18 3.11. Climatic data collection 19 3.12. Statistical analysis 19 CHAPTER 4 Results 22 4.1. Characterization of above ground ectomycorrhizal fungi 22 4.2. Identification of ectomycorrhizal host 184 4.3. Characterization of non ectomycorrhizal fruit bodies 186 4.4. Characterization of saprobic fungi found from fruit bodies 188 4.5. Characterization of below ground ectomycorrhizal fungi 189 4.6. Characterization of below ground non ectomycorrhizal fungi 193 4.7. Identification of host taxa from ectomycorrhizal morphotypes 195 4.8.