Characterization of Sympatric [I]Platanthera Bifolia[I]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phytogeographical Analysis and Ecological Factors of the Distribution of Orchidaceae Taxa in the Western Carpathians (Local Study)
plants Article Phytogeographical Analysis and Ecological Factors of the Distribution of Orchidaceae Taxa in the Western Carpathians (Local study) Lukáš Wittlinger and Lucia Petrikoviˇcová * Department of Geography and Regional Development, Faculty of Natural Sciences, Constantine the Philosopher University in Nitra, 94974 Nitra, Slovakia; [email protected] * Correspondence: [email protected]; Tel.: +421-907-3441-04 Abstract: In the years 2018–2020, we carried out large-scale mapping in the Western Carpathians with a focus on determining the biodiversity of taxa of the family Orchidaceae using field biogeographical research. We evaluated the research using phytogeographic analysis with an emphasis on selected ecological environmental factors (substrate: ecological land unit value, soil reaction (pH), terrain: slope (◦), flow and hydrogeological productivity (m2.s−1) and average annual amounts of global radiation (kWh.m–2). A total of 19 species were found in the area, of which the majority were Cephalenthera longifolia, Cephalenthera damasonium and Anacamptis morio. Rare findings included Epipactis muelleri, Epipactis leptochila and Limodorum abortivum. We determined the ecological demands of the abiotic environment of individual species by means of a functional analysis of communities. The research confirmed that most of the orchids that were studied occurred in acidified, calcified and basophil locations. From the location of the distribution of individual populations, it is clear that they are generally arranged compactly and occasionally scattered, which results in ecological and environmental diversity. During the research, we identified 129 localities with the occurrence of Citation: Wittlinger, L.; Petrikoviˇcová, L. Phytogeographical Analysis and 19 species and subspecies of orchids. We identify the main factors that threaten them and propose Ecological Factors of the Distribution specific measures to protect vulnerable populations. -

The Moth Pollinators of Greater Butterfly Orchids Platanthera
JOURNAL of the HARDY ORCHID SOCIETY Vol. 11 No. 1 (71) January 2014 The Moth Pollinators of Greater Butterfly Orchids Platanthera chlorantha in Central Scotland Roy Sexton With essential help from the following moth recorders: Stuart Bence, Claire Bird, Lorna Blackmore, Tim Brain, Michael Christie, Jennifer Davidson, Bob Dawson, John Knowler, Barbara Macritchie, John Oates, Tony Rogers, Melissa Shaw. In his book ‘T he Various Contrivances by which Orchids are Fertilised by Insects ’ Charles Darwin (1862; 1877) speculated that the Greater Butterfly Orchid (GBO) was pollinated by large night-flying moths. He based his proposal on the following observations: i) the flowers were white and so would show up at night ii) the floral scent to attract pollinators was produced nocturnally iii) the sugary nectar to reward insect visitors was found at the end of a 26mm long conical nectary or spur. This could only be reached by insects with long tongues like butterflies or moths. Careful examination of the structure of the flowers led Darwin to propose that when moths drank nectar the club-shaped masses of pollen positioned either side of the spur entrance became glued to their large com - pound eyes. He speculated that the pollen mass was repositioned as it dried and twist - ed so that it was deposited on the stigmas of the flowers which the moth visited subse - quently. Naturalists soon caught moths with GBO pollen on their eyes consistent with this hypothesis, but it was the meticulous studies of Anders Nilsson (1978) a century later that provided detailed scientific sup - port. It is his Swedish observations that I have attempted to confirm on Scottish plants. -

Girosnotizie 15
GIROS Notizie n. 15 - 2000 GIROS NOTIZIE G.I.R.O.S. Notiziario per i Soci Gruppo Italiano per la Ricerca sulle o Orchidee Spontanee Anno 2000 - N 15 web: http://astrpi.difi.unipi.it/Orchids/Giros.html http://astrpi.difi.unipi.it/Orchids-NEW Redazione e impaginazione a cura di: e-mail: [email protected] Bruno Barsella [email protected] ([email protected]) Mauro Biagioli ([email protected]) Sede legale: Paolo Grünanger ([email protected]) Via Testi, 7 - 48018 FAENZA (RA) Giuliano Pacifico Tel# 0546/30833 (Paolo Liverani) ([email protected]) Comitato Scientifico: Segreteria: Carlo Del Prete ([email protected]) Via Rosi, 21 - 55100 LUCCA (LU) Paolo Grünanger ([email protected]) Tel# 0583/492169 (Marcello Pieruccini) Giorgio Perazza Quota sociale 2000: L. 50.000 Grafica copertine: o Patrizia Cini e da versare sul c.c.p. n 13552559 intestato a: Bruno Barsella Gruppo Micologico M. Danesi A.M.B. 55029 - Ponte a Moriano - Lucca Sulla copertina: Cariche sociali per il triennio 2000-2002 Epipogium aphyllum Swartz foto di Rolando Romolini Consiglio Direttivo: Paolo Liverani (Presidente) Bruno Barsella (Vicepresidente) Marcello Pieruccini (Segretario) Stivi Betti (Tesoriere) M. Elisabetta Aloisi Masella NOTA DELLA REDAZIONE: Mauro Biagioli Rolando Romolini Ringraziamo i numerosi soci che hanno contribuito alla realizzazione di questo Sindaci Revisori: n u m e ro di “GIROS Notizie”. Rinnoviamo l’invito a collaborare alla Fulvio Fiesoli stesura dei notiziari inviando alla Claudio Merlini (Coordinatore) Redazione articoli, fotografie e suggeri - Michele Petroni menti. Nella ultima pagina di questo numero si vedano le norme redazionali da seguire per sottoporre manoscritti, disegni e immagini. -

The Hardy Orchid Society Newsletter
The Hardy Orchid Society Newsletter No. 18 October 2000 The Hardy Orchid Society Committee is… President: Paul Harcourt Davies, 128 Church Road, Sandford on Thames, Oxford, OX4 4TB Chairman: Adrian Blundell, 4 Raby Cottages, Sheinton Road, Cressage, Shrewsbury, SY5 6BX Vice-Chairman: Richard Manuel, Wye View Cottage, Leys Hill, Ross-on-Wye, Hereford- shire, HR9 5QU Secretary: Sarah Marks, 83 Ladysmith, East Gomeldon, Salisbury, Wilts, SP4 6LE Treasurer: Tony Beresford, Pound Farm, Wearne, Langport, Somerset, TA10 0QJ Membership Secretary: Nick Storer, 17 Orchard Close, Lymm, Cheshire, WA13 9HH Show Secretary: Tony Hughes, 8 Birchwood Rd., Leigh Sinton, Malvern, Worcs, WR14 1LD Newsletter Editor: Moira Tarrant, Bumby’s, Fox Rd., Mashbury, Chelmsford, CM1 4TJ Meetings Secretary: Colin Clay, 14 Cromwell Place, Lighthorne Heath, Leamington Spa, CV33 9TG Conservation Officer: Alan Dash, Lower Lakes, Suckley Knowle, Whitbourne, Worcs, WR6 5RH Ordinary Member (publicity): Simon Tarrant, Bumby’s, Fox Rd., Mashbury, Chelmsford, CM1 4TJ Ordinary Member (Newsletter Dist.): Bill Temple, Primrose Cottage, Hanney Rd., Ste- venton, Oxon, OX13 6AP Ordinary Member (Seed & Fungus Bank): Ted Weeks, 74 Over Lane, Almondsbury, Bristol, BS32 4BT Co-opted Member (BOC Rep.): Richard Nicol, 1364 Evesham Rd., Astwood Bank, Red- ditch, Worcs, B96 6BD Contents P.3 Autumn Meeting 2000 P.4 HOS Millennium Photo Competition P.5 Orchid & Sightseeing Tour of the West Coast of USA by Bill Temple P.7 A Glorious Day - in Spite of the Rain by Tony and Diana Hughes P.10 An Orchidaceous Bacchanalia by Richard Manuel P.13 Orchid Hunting in Fife by Patrick Marks P.17 Members Ask P.19 Orchids in the News Enclosed with this Newsletter: Application Form for the Autumn Meeting Cover illustration: Calopogon tuberosus by Carol Dash HOS Newsletter 18, October 2000 Autumn Meeting, 2000 Colin Clay, Meetings Secretary The next meeting will be on Sunday 29th October 2000 at Horticulture Research International, Wellesbourne, near Warwick. -

20120620144528 1897.Pdf
Annals of Botany 104: 431–445, 2009 doi:10.1093/aob/mcp089, available online at www.aob.oxfordjournals.org Molecular phylogenetics and morphological reappraisal of the Platanthera clade (Orchidaceae: Orchidinae) prompts expansion of the generic limits of Galearis and Platanthera Richard M. Bateman1,*, Karen E. James2, Yi-Bo Luo3, Robert K. Lauri4, Timothy Fulcher1, Phillip J. Cribb1 and Mark W. Chase1 1Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, UK, 2Department of Botany, Natural History Museum, Cromwell Road, London SW7 5BD, UK, 3Institute of Botany, Chinese Academy of Sciences, Xiangshan, Beijing, 100093, China and 4Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, CA 91711, USA Received: 22 July 2008 Returned for revision: 4 December 2008 Accepted: 30 March 2009 Published electronically: 21 April 2009 † Background and Aims The Platanthera clade dominates the North American orchid flora and is well represented in eastern Asia. It has also generated some classic studies of speciation in Platanthera sections Platanthera and Limnorchis. However, it has proved rich in taxonomic controversy and near-monotypic genera. The clade is reviewed via a new molecular phylogenetic analysis and those results are combined with brief reconsideration of morphology in the group, aiming to rationalize the species into a smaller number of larger monophyletic genera and sections. † Methods Nuclear ribosomal internal transcribed spacer (ITS) sequences were obtained from 86 accessions of 35 named taxa, supplemented from GenBank with five accessions encompassing a further two named taxa. † Key Results Using Pseudorchis as outgroup, and scoring indels, the data matrix generated 30 most-parsimo- nious trees that differed in the placement of two major groups plus two closely related species. -

Rchb. in the Republic of Adygea
Ecologica Montenegrina 23: 20-26 (2019) This journal is available online at: www.biotaxa.org/em Modern Distribution and Ecological-phytocenotic Features of Platanthera chlorantha (Cust.) Rchb. in the Republic of Adygea AMINET E. SHADGE*, EMILIA A. SIROTYUK, GALINA N. GUNINA & VLADIMIR V. KHIRYANOV Maykop State Technological University, 385000, Maykop, Russia *Corresponding author: E-mail: [email protected] Received 15 June 2019 │ Accepted by V. Pešić: 20 September 2019 │ Published online 16 October 2019 Abstract During the monitoring of rare plant species in the Maykop region of the Republic of Adygea, known and discovered new locations of P. chlorantha were confirmed, for which the height above sea level and geographical coordinates were determined. The identification of the species to a certain range of altitudes above sea level and types of phytocenoses was revealed. Ongenetic states, ontogenetic structure and intraspecific variability of P. chlorantha were studied in populations. Key words: locations, populations, ontogenetic states, ontogenetic structure, intraspecific variability. Introduction In connection with the growing negative impact of human activity on the environment, the problem of preserving the biodiversity of the planet becomes especially acute. One of the most vulnerable components of plant communities are species of the Orchidaceae Juss. Family, which is associated primarily with their bioecological features: highly specialized entomophilia; undifferentiated embryo; the lack of spare substances in the seed, which contributes to the long-term formation of the protocorm and makes it dependent on mycorrhizal fungi; low competitiveness; narrow ecological amplitude and high sensitivity to anthropogenic influences. The listed features of orchids cause their rarity in natural habitats. The most rare and endangered species in the territory of Adygea are listed in the Red Book of the Republic of Adygea (2012). -

Download Download
Firenze University Press Caryologia www.fupress.com/caryologia International Journal of Cytology, Cytosystematics and Cytogenetics Comparison of the Evolution of Orchids with that of Bats Citation: A. Lima-de-Faria (2020) Comparison of the Evolution of Orchids with that of Bats. Caryologia 73(2): 51-61. doi: 10.13128/caryologia-891 Antonio Lima-de-Faria Received: February 12, 2020 Professor Emeritus of Molecular Cytogenetics, Lund University, Lund, Sweden E-mail: [email protected] Accepted: April 16, 2020 Published: July 31, 2020 Abstract. The evolution of orchids and bats is an example of DNA’s own evolution which has resulted in structures and functions which are not necessarily related to any Copyright: © 2020 A. Lima-de-Faria. obvious advantage to the organism. The flowers of orchids resemble: humans, apes, liz- This is an open access, peer-reviewed article published by Firenze University ards, frogs and even shoes. The faces of bats resemble plant leaves but also horseshoes. Press (http://www.fupress.com/caryo- These similarities are not accidental because they emerge repeatedly in different gen- logia) and distributed under the terms era and different families. This evolutionary situation bewildered botanists and zoolo- of the Creative Commons Attribution gists for many years, but is now elucidated by the molecular unification of plants and License, which permits unrestricted animals derived from the following evidence: (1) Contrary to expectation, plant and use, distribution, and reproduction animal cells (including those of humans) could be fused and the human chromosomes in any medium, provided the original were seen dividing in the plant cytoplasm. (2) Orchids, bats and humans have about author and source are credited. -

Pseudorchis Albida (L.) Á
Pseudorchis albida (L.) Á. and D. Löve Small White Orchid As the common name suggests, Pseudorchis albida is a diminutive orchid with tiny, creamy- white, scented flowers that attract a variety of day-flying insects. It is able to tolerate a wide range of ecological conditions, having a pH range of between 4 and 7, but is most often found in low fertility mesotrophic grasslands and acid heathlands, and more rarely in base- rich mires, limestone heath, and cliff ledges. It is most widespread in the north and west of Scotland but has experienced substantial declines across its range, particularly in Ireland, England and Wales. Pseudorchis albida is assessed as Vulnerable in GB and Critically Endangered in Wales. ©Joan Canals IDENTIFICATION having stomata above (only on the underside of the leaf in P. albida), G. repens by the presence of petioles and C. viride by Pseudorchis albida is a diminutive orchid with a dense spike the absence of longitudinal streaking (Poland & Clements, of very small, creamy-white flowers (2-4 mm across) with a 2009). In Britain, hybrids with Gymnadenia borealis have deeply three-lobed lip. Its leaves have translucent longitudinal been widely reported from Scotland and northern England streaks, are glossy green above, silvery below, and have a and with Dactylorhiza maculata in Skye and Orkney. hooded tip (Poland & Clements, 2009). Throughout its range Reported hybrids with Platanthera chlorantha are now P. albida is probably overlooked due to its small stature, short thought to be aberrant forms of the latter. flowering period, erratic appearance and tendency to form small populations (Duffey et al., 2008). -
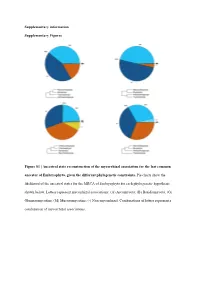
Ancestral State Reconstruction of the Mycorrhizal Association for the Last Common Ancestor of Embryophyta, Given the Different Phylogenetic Constraints
Supplementary information Supplementary Figures Figure S1 | Ancestral state reconstruction of the mycorrhizal association for the last common ancestor of Embryophyta, given the different phylogenetic constraints. Pie charts show the likelihood of the ancestral states for the MRCA of Embryophyta for each phylogenetic hypothesis shown below. Letters represent mycorrhizal associations: (A) Ascomycota; (B) Basidiomycota; (G) Glomeromycotina; (M) Mucoromycotina; (-) Non-mycorrhizal. Combinations of letters represent a combination of mycorrhizal associations. Austrocedrus chilensis Chamaecyparis obtusa Sequoiadendron giganteum Prumnopitys taxifolia Prumnopitys Prumnopitys montana Prumnopitys Prumnopitys ferruginea Prumnopitys Araucaria angustifolia Araucaria Dacrycarpus dacrydioides Dacrycarpus Taxus baccata Podocarpus oleifolius Podocarpus Afrocarpus falcatus Afrocarpus Ephedra fragilis Nymphaea alba Nymphaea Gnetum gnemon Abies alba Abies balsamea Austrobaileya scandens Austrobaileya Abies nordmanniana Thalictrum minus Thalictrum Abies homolepis Caltha palustris Caltha Abies magnifica ia repens Ranunculus Abies religiosa Ranunculus montanus Ranunculus Clematis vitalba Clematis Keteleeria davidiana Anemone patens Anemone Tsuga canadensis Vitis vinifera Vitis Tsuga mertensiana Saxifraga oppositifolia Saxifraga Larix decidua Hypericum maculatum Hypericum Larix gmelinii Phyllanthus calycinus Phyllanthus Larix kaempferi Hieronyma oblonga Hieronyma Pseudotsuga menziesii Salix reinii Salix Picea abies Salix polaris Salix Picea crassifolia Salix herbacea -

Diversity and Distribution of Floral Scent
The Botanical Review 72(1): 1-120 Diversity and Distribution of Floral Scent JETTE T. KNUDSEN Department of Ecology Lund University SE 223 62 Lund, Sweden ROGER ERIKSSON Botanical Institute GOteborg University Box 461, SE 405 30 GOteborg, Sweden JONATHAN GERSHENZON Max Planck Institute for Chemical Ecology Hans-KnOll Strasse 8, 07745 ,lena, Germany AND BERTIL ST,~HL Gotland University SE-621 67 Visby, Sweden Abstract ............................................................... 2 Introduction ............................................................ 2 Collection Methods and Materials ........................................... 4 Chemical Classification ................................................... 5 Plant Names and Classification .............................................. 6 Floral Scent at Different Taxonomic Levels .................................... 6 Population-Level Variation .............................................. 6 Species- and Genus-Level Variation ....................................... 6 Family- and Order-Level Variation ........................................ 6 Floral Scent and Pollination Biology ......................................... 9 Floral Scent Chemistry and Biochemistry ...................................... 10 Floral Scent and Evolution ................................................. 11 Floral Scent and Phylogeny ................................................ 12 Acknowledgments ....................................................... 13 Literature Cited ........................................................ -

Characterization of Sympatric Platanthera Bifolia and Platanthera Chlorantha (Orchidaceae) Populations with Intermediate Plants
Characterization of sympatric Platanthera bifolia and Platanthera chlorantha (Orchidaceae) populations with intermediate plants Fabiana Esposito1, Nicolas J. Vereecken2, Maddalena Gammella3, Rosita Rinaldi3, Pascal Laurent4 and Daniel Tyteca1 1 Earth and Life Institute—Biodiversity Research Centre, Université Catholique de Louvain, Louvain-la-Neuve, Belgium 2 Agroecology Lab, Brussels Bioengineering School, Université libre de Bruxelles (ULB), Brussels, Belgium 3 Department of Biology, University of Naples Federico II, Napoli, Italy 4 Unit of General Chemistry, Université Libre de Bruxelles, Brussels, Belgium ABSTRACT Platanthera bifolia and P. chlorantha are terrestrial and rewarding orchids with a wide Eurasian distribution. Although genetically closely related, they exhibit significant morphological, phenological and ecological differences that maintain reproductive isolation between the species. However, where both species co-occur, individuals with intermediate phenotypic traits, often considered as hybrids, are frequently observed. Here, we combined neutral genetic markers (AFLPs), morphometrics and floral scent analysis (GC-MS) to investigate two mixed Platanthera populations where morphologically intermediate plants were found. Self-pollination experiments revealed a low level of autogamy and artificial crossings combined with assessments of fruit set and seed viability, showed compatibility between the two species. The results of the genetic analyses showed that morphologically intermediate plants had similar genetic patterns -

Noctuid Moths As Potential Hybridization Agents for Platanthera Orchids
LANKESTERIANA 17(3): 383–393. 2017. doi: http://dx.doi.org/10.15517/lank.v17i3.31576 NOCTUID MOTHS AS POTENTIAL HYBRIDIZATION AGENTS FOR PLATANTHERA ORCHIDS FABIANA ESPOSITO1, THOMAS MERCKX2 & DANIEL TYTECA1,3 1 Orchid Research Group, Biodiversity Research Centre, Earth and Life Institute, Université catholique de Louvain (UCL), Croix du Sud 4-5 (L7.07.04), B-1348 Louvain-la-Neuve, Belgium 2 Behavioural Ecology and Conservation Group, Biodiversity Research Centre, Earth and Life Institute, Université catholique de Louvain (UCL), Croix du Sud 4-5 (L7.07.04), B-1348 Louvain-la-Neuve, Belgium 3 Corresponding author: e-mail: [email protected] ABSTRACT. Zoophilous flowering plants communicate with pollinators to ensure pollen transfer. Pin-pointing which species are effective pollinators is not only essential to better understand plant-pollinator networks, but equally so to better understand the potential of hybridization in plant systems, such as in orchids. As a case study, we studied two sympatric populations of the congeneric orchids Platanthera bifolia and P. chlorantha in order to assess their nocturnal pollinators by checking which moth species carried pollinaria, and of which orchid species. Moths carrying Platanthera pollinaria were photographed and identified. The carried pollinaria were identified and counted, and their attachment position on the moth’s head was scored. Based on these observations we show that three species of noctuid moths visited the Platanthera inflorescences. Although Noctua pronuba visited P. chlorantha, only Cucullia umbratica and Autographa gamma turned out to be potential pollinators for both orchid species. As such, we here demonstrate that the latter two noctuids have high potential to facilitate hybridization among these two orchid species, especially so in sympatric populations.