Effects of Breed of Sheep and Dietary Onions on Bitterweed (Hymenoxys Odorata DC) Toxicity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UPDATED 18Th February 2013
7th February 2015 Welcome to my new seed trade list for 2014-15. 12, 13 and 14 in brackets indicates the harvesting year for the seed. Concerning seed quantity: as I don't have many plants of each species, seed quantity is limited in most cases. Therefore, for some species you may only get a few seeds. Many species are harvested in my garden. Others are surplus from trade and purchase. OUT: Means out of stock. Sometimes I sell surplus seed (if time allows), although this is unlikely this season. NB! Cultivars do not always come true. I offer them anyway, but no guarantees to what you will get! Botanical Name (year of harvest) NB! Traditional vegetables are at the end of the list with (mostly) common English names first. Acanthopanax henryi (14) Achillea sibirica (13) Aconitum lamarckii (12) Achyranthes aspera (14, 13) Adenophora khasiana (13) Adenophora triphylla (13) Agastache anisata (14,13)N Agastache anisata alba (13)N Agastache rugosa (Ex-Japan) (13) (two varieties) Agrostemma githago (13)1 Alcea rosea “Nigra” (13) Allium albidum (13) Allium altissimum (Persian Shallot) (14) Allium atroviolaceum (13) Allium beesianum (14,12) Allium brevistylum (14) Allium caeruleum (14)E Allium carinatum ssp. pulchellum (14) Allium carinatum ssp. pulchellum album (14)E Allium carolinianum (13)N Allium cernuum mix (14) E/N Allium cernuum “Dark Scape” (14)E Allium cernuum ‘Dwarf White” (14)E Allium cernuum ‘Pink Giant’ (14)N Allium cernuum x stellatum (14)E (received as cernuum , but it looks like a hybrid with stellatum, from SSE, OR KA A) Allium cernuum x stellatum (14)E (received as cernuum from a local garden centre) Allium clathratum (13) Allium crenulatum (13) Wild coll. -

Special-Status Plants and Invasive/Noxious Weeds Technical Report
SACRAMENTO MUNICIPAL UTILITY DISTRICT UPPER AMERICAN RIVER PROJECT (FERC NO. 2101) SPECIAL-STATUS PLANTS AND INVASIVE/NOXIOUS WEEDS TECHNICAL REPORT Prepared by: Devine Tarbell & Associates, Inc. Sacramento, California Prepared for: Sacramento Municipal Utility District Sacramento, California JULY 2004 Sacramento Municipal Utility District Upper American River Project FERC Project No. 2101 TABLE OF CONTENTS Section & Description Page 1.0 INTRODUCTION .............................................................................................................. 1 2.0 BACKGROUND ................................................................................................................ 2 2.1 Special-Status Plants Study Plan ............................................................................ 2 2.2 Invasive/Noxious Weeds Study Plan...................................................................... 3 2.3 Water Year Types................................................................................................... 4 2.4 Agency Requested Information .............................................................................. 5 3.0 METHODS ......................................................................................................................... 5 3.1 Special-Status Plants............................................................................................... 5 3.2 Noxious Weeds ....................................................................................................... 6 4.0 RESULTS .......................................................................................................................... -

Texas Prairie Dawn-Flower (Hymenoxys Texana) 5-Year Review
Texas prairie dawn-flower (Hymenoxys texana) 5-Year Review: Summary and Evaluation Photo credit: USFWS U.S. Fish and Wildlife Service Texas Coastal Ecological Services Field Office Houston, Texas Table of Contents ABBREVIATIONS ........................................................................................................................ 3 1.0 GENERAL INFORMATION .......................................................................................... 4 2.0 REVIEW ANALYSIS ...................................................................................................... 7 2.4 SYNTHESIS .................................................................................................................. 24 3.0 RESULTS....................................................................................................................... 25 4.0 RECOMMENDATIONS FOR FUTURE ACTIONS.................................................... 26 5.0 REFERENCES ............................................................................................................... 28 Appendix A ................................................................................................................................... 31 Recommendation resulting from the 5-Year Review: .................................................................. 34 Figures Figure 1 Current H. texana county occurrences ............................................................................. 9 Tables Table 1 Renaming of species historically associated with H. texana .......................................... -

Illinois Bundleflower (Desmanthus Illinoensis) Story by Alan Shadow, Manager USDA-NRCS East Texas Plant Materials Center Nacogdoches, Texas
Helping People Help The Land September/October 2011 Issue No. 11 The Reverchon Naturalist Recognizing the work of French botanist Julien Reverchon, who began collecting throughout the North Central Texas area in 1876, and all the botanists/naturalists who have followed ... Drought, Heat and Native Trees ranging from simple things like more extensive root systems, to more drastic measures like pre- Story by Bruce Kreitler mature defoliation, what they actually have little Abilene, Texas defense against is a very prolonged period of no appreciable water supply. nybody that has traveled in Texas this year A will have noticed that not only most of the By the way, even though they are usually the land browned out, but also if you look at the trees same species, there is a difference in landscape in the fields and beside the roads, they aren't trees and native trees, which are untended plants looking so good either. It doesn't take a rocket that have to fend for themselves. While they are scientist to realize that extreme high temperatures indeed the same basic trees, the differences be- combined with, and partially caused by, drought tween the environments that they live in are huge are hard on trees. and thus overall general environmental factors such as drought, temperature, and insect infesta- Since I'm pretty sure that most of the people read- tions act on them differently. For the purposes of ing this article understand very well that drought this article, I'm referring to trees that are on their is a problem for trees, the question isn't is the pre- own, untended for their entire lives in fields, pas- sent drought going to have an effect on trees, but tures, forests, or just wherever nature has placed rather, what are the present effects of the drought them and refer to them as native trees. -

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -

A POCKET GUIDE to Kansas Red Hills Wildflowers
A POCKET GUIDE TO Kansas Red Hills Wildflowers ■ ■ ■ ■ By Ken Brunson, Phyllis Scherich, Chris Berens, and Carl Jarboe Sponsored by Chickadee Checkoff, Westar Energy Green Team, The Nature Conservancy in Kansas, Kansas Grazing Lands Coalition and Comanche Pool Prairie Resource Foundation Published by the Friends of the Great Plains Nature Center Table of Contents • Introduction • 2 Blue/Purple ■ Oklahoma Phlox • 6 ■ Twist-flower • 7 ■ Blue Funnel-lily • 8 ■ Purple Poppy Mallow • 9 ■ Prairie Spiderwort • 10 ■ Purple Ground Cherry • 11 ■ Purple Locoweed • 12 ■ Stevens’ Nama • 13 ■ Woolly Locoweed • 14 Easter Daisy ■ Wedge-leaf Frog Fruit • 15 ©Phyllis Scherich ■ Silver-leaf Nightshade • 16 Cover Photo: Bush ■ Prairie Gentian • 17 Morning-glory ■ Woolly Verbena • 18 ©Phyllis Scherich ■ Stout Scorpion-weed • 19 Pink/Red ■ Rayless Gaillardia • 20 ■ Velvety Gaura • 21 ■ Western Indigo • 22 ■ Pincushion Cactus • 23 ■ Scarlet Gaura • 24 ■ Bush Morning-glory • 25 ■ Indian Blanket Flower • 26 ■ Clammy-weed • 27 ■ Goat’s Rue • 28 White/Cream Easter Daisy • 29 Old Plainsman • 30 White Aster • 31 Western Spotted Beebalm • 32 Lazy Daisy • 33 Prickly Poppy • 34 White Beardtongue • 35 Yucca • 36 White Flower Ipomopsis • 37 Stenosiphon • 38 White Milkwort • 39 Annual Eriogonum • 40 Devil’s Claw • 41 Ten-petal Mentzelia • 42 Yellow/Orange ■ Slender Fumewort • 43 ■ Bladderpod • 44 ■ Indian Blanket Stiffstem Flax • 45 Flower ■ Lemon Paintbrush • 46 ©Phyllis Scherich ■ Hartweg Evening Primrose • 47 ■ Prairie Coneflower • 48 ■ Rocky Mountain -

Lakeside Daisy Hymenoxys Herbacea
COSEWIC Assessment and Status Report on the Lakeside Daisy Hymenoxys herbacea in Canada THREATENED 2002 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION DES ENDANGERED WILDLIFE IN ESPÈCES EN PÉRIL CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: Please note: Persons wishing to cite data in the report should refer to the report (and cite the author(s)); persons wishing to cite the COSEWIC status will refer to the assessment (and cite COSEWIC). A production note will be provided if additional information on the status report history is required. COSEWIC 2002. COSEWIC assessment and status report the lakeside daisy Hymenoxys herbacea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 24 pp. Campbell, L.B. Husband and M.J. Oldham 2002. COSEWIC status report on the lakeside daisy Hymenoxys herbacea in Canada, in COSEWIC assessment and status report the lakeside daisy Hymenoxys herbacea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-24 pp. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Évaluation et Rapport de situation du COSEPAC sur l’hyménoxys herbacé (Hymenoxys herbacea) au Canada Cover illustration: Lakeside Daisy — Illustration by Jack Wellington Her Majesty the Queen in Right of Canada 2003 Catalogue No. -

Biological Control of Rangeland Weeds
Reprinted with permission from: Noxious Range Weeds. 1991. Chapter 9. pp. 83-102. Published by: Westview Press, Boulder, San Francisco, & Oxford. Biological control of rangeland weeds P. C. QUIMBY, JR., W. L. BRUCKART, C. J. DELOACH, LLOYD KNUTSON, and M. H. RALPHS Abstract: Weedy forbs and brush cost America’s range managers at least $1.7 bil- lion/year. Biological controls, or “the planned use of living organisms to reduce the vigor, reproductive capacity, density, or effect of weeds,” should be considered and included in pragmatic integrated weed manage- ment systems for rangelands. Various approaches to biocontrol under that definition are discussed. These include foreign exploration and introduc- tion of exotic insects, notes, and plant pathogens as biocontrol agents; augmentation of native biocontrol agents, especially plant pathogens; grazing systems; and positive and aversion conditioning for various classes of livestock to use against troublesome weeds or brush or to avoid palatable poisonous weeds. USDA’s Agricultural Research Service has at least nine laboratories, worldwide, devoted to research on various aspects of biocontrol of exotic and native rangeland weeds. The usual goal of bio- control is to improve ecological systems by using biotic agents to restore target plant species to lesser competitive intensities or to negate their ef- fects so that they do not overwhelm plant communities or cause damage to livestock. The usual results of biocontrol are: improved agricultural pro- duction, improved ecosystem functions and status in terms of species rich- ness and diversity of plant and animal communities, and improved protection of rare species. Regardless of whether target weeds are intro- duced or native, researchers must make balanced evaluations of risks, benefits, and the potential for success in developing biological control programs. -
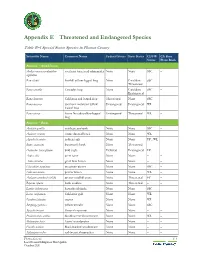
Appendix E Threatened and Endangered Species
Appendix E Threatened and Endangered Species Table E–1 Special Status Species in Plumas County Scientific Name Common Name Federal Status State Status CDFW CA Rare Status Plant Rank Animals – Amphibians Ambystoma macrodactylum southern long-toed salamander None None SSC – sigillatum Rana boylii foothill yellow-legged frog None Candidate SSC – Threatened Rana cascadae Cascades frog None Candidate SSC – Endangered Rana draytonii California red-legged frog Threatened None SSC – Rana muscosa southern mountain yellow- Endangered Endangered WL – legged frog Rana sierrae Sierra Nevada yellow-legged Endangered Threatened WL – frog Animals – Birds Accipiter gentilis northern goshawk None None SSC – Accipiter striatus sharp-shinned hawk None None WL – Aquila chrysaetos golden eagle None None FP ; WL – Buteo swainsoni Swainson's hawk None Threatened – – Haliaeetus leucocephalus bald eagle Delisted Endangered FP – Ardea alba great egret None None – – Ardea herodias great blue heron None None – – Charadrius montanus mountain plover None None SSC – Falco mexicanus prairie falcon None None WL – Antigone canadensis tabida greater sandhill crane None Threatened FP – Riparia riparia bank swallow None Threatened – – Lanius ludovicianus loggerhead shrike None None SSC – Larus californicus California gull None None WL – Pandion haliaetus osprey None None WL – Setophaga petechia yellow warbler None None SSC – Spizella breweri Brewer's sparrow None None – – Phalacrocorax auritus double-crested cormorant None None WL – Melanerpes lewis Lewis' woodpecker None -

Vascular Plant Species with Documented Or Recorded Occurrence in Placer County
A PPENDIX II Vascular Plant Species with Documented or Reported Occurrence in Placer County APPENDIX II. Vascular Plant Species with Documented or Reported Occurrence in Placer County Family Scientific Name Common Name FERN AND FERN ALLIES Azollaceae Mosquito fern family Azolla filiculoides Pacific mosquito fern Dennstaedtiaceae Bracken family Pteridium aquilinum var.pubescens Bracken fern Dryopteridaceae Wood fern family Athyrium alpestre var. americanum Alpine lady fern Athyrium filix-femina var. cyclosorum Lady fern Cystopteris fragilis Fragile fern Polystichum imbricans ssp. curtum Cliff sword fern Polystichum imbricans ssp. imbricans Imbricate sword fern Polystichum kruckebergii Kruckeberg’s hollyfern Polystichum lonchitis Northern hollyfern Polystichum munitum Sword fern Equisetaceae Horsetail family Equisetum arvense Common horsetail Equisetum hyemale ssp. affine Scouring rush Equisetum laevigatum Smooth horsetail Isoetaceae Quillwort family Isoetes bolanderi Bolander’s quillwort Isoetes howellii Howell’s quillwort Isoetes orcuttii Orcutt’s quillwort Lycopodiaceae Club-moss family Lycopodiella inundata Bog club-moss Marsileaceae Marsilea family Marsilea vestita ssp. vestita Water clover Pilularia americana American pillwort Ophioglossaceae Adder’s-tongue family Botrychium multifidum Leathery grapefern Polypodiaceae Polypody family Polypodium hesperium Western polypody Pteridaceae Brake family Adiantum aleuticum Five-finger maidenhair Adiantum jordanii Common maidenhair fern Aspidotis densa Indian’s dream Cheilanthes cooperae Cooper’s -

Wyoming FWS Endangered Species List
United States Department of the Interior FISH AND WILDLIFE SERVICE Ecological Services 5353 Yellowstone Road, Suite 308A Cheyenne, Wyoming 82009 In Reply Refer To: ES-6141 1/W.32/WY07SL0361 JAN 1 '2008 Mr. Ron C. Linton Senior Groundwater Hydrologist/Project Manager U.S. Nuclear Regulatory Commission Office of Federal and State Materials And Environmental Management Programs Mail Stop T-8F5 11545 Rockville Pike Rockville, Maryland 20852-2738 Dear Mr. Linton: Please find enclosed the U.S. Fish and Wildlife Service's (Service) current list of endangered, threatened, and candidate species which may occur within the State of Wyoming. This list is provided as a general reference for the U.S. Nuclear Regulatory Commission (NRC) to use when evaluating actions under the Endangered Species Act of 1973, as amended (Act) (16 U.S.C. 1531 et seq.). We have revised our previous species list to reflect the recovery and delisting of the bald eagle (Haliaeetusleucocephalus). On July 9, 2007, the Service published a Federal Register notice (72 FR 37346) announcing that the bald eagle would be removed from the list of threatened and endangered species under the Act on August 8, 2007. However, the protections provided to the bald eagle under the Bald and Golden Eagle Protection Act, 16 U.S.C. 668 (BGEPA) and the Migratory Bird Treaty Act, 16 U.S.C. 703 (MBTA) will remain in place. The term "disturb" under the BGEPA has recently been defined as: "to agitate or bother a bald or golden eagle to a degree that causes, or is likely to cause, based on the best scientific information available, (1) injury to an eagle, (2) a decrease in its productivity, by substantially interfering with normal breeding, feeding, or sheltering behavior, or (3) nest abandonment, by substantially interfering with normal breeding, feeding, or sheltering behavior (72 FR 31332). -

Plant List Lomatium Mohavense Mojave Parsley 3 3 Lomatium Nevadense Nevada Parsley 3 Var
Scientific Name Common Name Fossil Falls Alabama Hills Mazourka Canyon Div. & Oak Creeks White Mountains Fish Slough Rock Creek McGee Creek Parker Bench East Mono Basin Tioga Pass Bodie Hills Cicuta douglasii poison parsnip 3 3 3 Cymopterus cinerarius alpine cymopterus 3 Cymopterus terebinthinus var. terebinth pteryxia 3 3 petraeus Ligusticum grayi Gray’s lovage 3 Lomatium dissectum fern-leaf 3 3 3 3 var. multifidum lomatium Lomatium foeniculaceum ssp. desert biscuitroot 3 fimbriatum Plant List Lomatium mohavense Mojave parsley 3 3 Lomatium nevadense Nevada parsley 3 var. nevadense Lomatium rigidum prickly parsley 3 Taxonomy and nomenclature in this species list are based on Lomatium torreyi Sierra biscuitroot 3 western sweet- the Jepson Manual Online as of February 2011. Changes in Osmorhiza occidentalis 3 3 ADOXACEAE–ASTERACEAE cicely taxonomy and nomenclature are ongoing. Some site lists are Perideridia bolanderi Bolander’s 3 3 more complete than others; all of them should be considered a ssp. bolanderi yampah Lemmon’s work in progress. Species not native to California are designated Perideridia lemmonii 3 yampah with an asterisk (*). Please visit the Inyo National Forest and Perideridia parishii ssp. Parish’s yampah 3 3 Bureau of Land Management Bishop Resource Area websites latifolia for periodic updates. Podistera nevadensis Sierra podistera 3 Sphenosciadium ranger’s buttons 3 3 3 3 3 capitellatum APOCYNACEAE Dogbane Apocynum spreading 3 3 androsaemifolium dogbane Scientific Name Common Name Fossil Falls Alabama Hills Mazourka Canyon Div. & Oak Creeks White Mountains Fish Slough Rock Creek McGee Creek Parker Bench East Mono Basin Tioga Pass Bodie Hills Apocynum cannabinum hemp 3 3 ADOXACEAE Muskroot Humboldt Asclepias cryptoceras 3 Sambucus nigra ssp.