Lakeside Daisy Hymenoxys Herbacea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Texas Prairie Dawn-Flower (Hymenoxys Texana) 5-Year Review
Texas prairie dawn-flower (Hymenoxys texana) 5-Year Review: Summary and Evaluation Photo credit: USFWS U.S. Fish and Wildlife Service Texas Coastal Ecological Services Field Office Houston, Texas Table of Contents ABBREVIATIONS ........................................................................................................................ 3 1.0 GENERAL INFORMATION .......................................................................................... 4 2.0 REVIEW ANALYSIS ...................................................................................................... 7 2.4 SYNTHESIS .................................................................................................................. 24 3.0 RESULTS....................................................................................................................... 25 4.0 RECOMMENDATIONS FOR FUTURE ACTIONS.................................................... 26 5.0 REFERENCES ............................................................................................................... 28 Appendix A ................................................................................................................................... 31 Recommendation resulting from the 5-Year Review: .................................................................. 34 Figures Figure 1 Current H. texana county occurrences ............................................................................. 9 Tables Table 1 Renaming of species historically associated with H. texana .......................................... -

Illinois Bundleflower (Desmanthus Illinoensis) Story by Alan Shadow, Manager USDA-NRCS East Texas Plant Materials Center Nacogdoches, Texas
Helping People Help The Land September/October 2011 Issue No. 11 The Reverchon Naturalist Recognizing the work of French botanist Julien Reverchon, who began collecting throughout the North Central Texas area in 1876, and all the botanists/naturalists who have followed ... Drought, Heat and Native Trees ranging from simple things like more extensive root systems, to more drastic measures like pre- Story by Bruce Kreitler mature defoliation, what they actually have little Abilene, Texas defense against is a very prolonged period of no appreciable water supply. nybody that has traveled in Texas this year A will have noticed that not only most of the By the way, even though they are usually the land browned out, but also if you look at the trees same species, there is a difference in landscape in the fields and beside the roads, they aren't trees and native trees, which are untended plants looking so good either. It doesn't take a rocket that have to fend for themselves. While they are scientist to realize that extreme high temperatures indeed the same basic trees, the differences be- combined with, and partially caused by, drought tween the environments that they live in are huge are hard on trees. and thus overall general environmental factors such as drought, temperature, and insect infesta- Since I'm pretty sure that most of the people read- tions act on them differently. For the purposes of ing this article understand very well that drought this article, I'm referring to trees that are on their is a problem for trees, the question isn't is the pre- own, untended for their entire lives in fields, pas- sent drought going to have an effect on trees, but tures, forests, or just wherever nature has placed rather, what are the present effects of the drought them and refer to them as native trees. -

Effects of Breed of Sheep and Dietary Onions on Bitterweed (Hymenoxys Odorata DC) Toxicity
Volume 29, 2014 - December Effects of Breed of Sheep and Dietary Onions on Bitterweed (Hymenoxys odorata DC) Toxicity E. S. Campbell1,3, T. R. Whitney2, C. A. Taylor, Jr.1, N. E. Garza1 1 Texas A&M AgriLife Research Center, Sonora, TX 76950 2 Texas A&M AgriLife Research and Extension Center, San Angelo, TX 76901 3 Corresponding author: [email protected] ACKNOWLEDGMENT Partial support for the research was provided by the Texas Food and Fiber Commission/ Texas Department of Agriculture, Austin, TX. Summary isonitrogenous diets consisted of alfalfa elicited a breed effect (P< 0.05) for pellets to provide 32 g DM/kg BW per serum measurements reflective of bitter- Bitterweed (Hymenoxys odorata day. Animals were group-fed onions for a weed toxicity; bilirubin, gamma-glu- DC) toxicity is a major cause of death 10-d preconditioning period, then tamyltransferase (GGT) and AST con- losses in Rambouillet sheep. This study penned and fed individually for study. centrations were greater (P ≤ 0.001) for compared the susceptibility of two Individual onion feeding commenced on DBBs than for Rambouillets. The AST, breeds [Rambouillet and Dorper × Bar- d 0 and continued through d 7. On d 3 of bilirubin, creatinine, GGT, and SUN bados Blackbelly (DBB)], of wool and onion feeding, lambs were dosed with an were clinically high for all treatments, hair sheep lambs to bitterweed toxicosis; aqueous slurry of dried bitterweed (0.25 including controls, indicating acute tox- and the potential for cull onions (Allium percent of BW, DM-basis) daily through icity. Feed refusals did not differ among cepa) to mitigate bitterweed toxicity. -

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -

Annotated Checklist of Vascular Flora, Bryce
National Park Service U.S. Department of the Interior Natural Resource Program Center Annotated Checklist of Vascular Flora Bryce Canyon National Park Natural Resource Technical Report NPS/NCPN/NRTR–2009/153 ON THE COVER Matted prickly-phlox (Leptodactylon caespitosum), Bryce Canyon National Park, Utah. Photograph by Walter Fertig. Annotated Checklist of Vascular Flora Bryce Canyon National Park Natural Resource Technical Report NPS/NCPN/NRTR–2009/153 Author Walter Fertig Moenave Botanical Consulting 1117 W. Grand Canyon Dr. Kanab, UT 84741 Sarah Topp Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 Editing and Design Alice Wondrak Biel Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 January 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientifi c community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifi cally credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. The Natural Resource Technical Report series is used to disseminate the peer-reviewed results of scientifi c studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service’s mission. The reports provide contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. -

Recovery Strategy for the Lakeside Daisy (Tetraneuris Herbacea) in Ontario
Photo: C.D. Jones, NHIC Archives Lakeside Daisy (Tetraneuris herbacea) in Ontario Ontario Recovery Strategy Series Recovery strategy prepared under the Endangered Species Act, 2007 2013 Ministry of Natural Resources About the Ontario Recovery Strategy Series � This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada. What is recovery? What’s next? Recovery of species at risk is the process by which the Nine months after the completion of a recovery strategy decline of an endangered, threatened, or extirpated a government response statement will be published species is arrested or reversed, and threats are which summarizes the actions that the Government of removed or reduced to improve the likelihood of a Ontario intends to take in response to the strategy. species’ persistence in the wild. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and What is a recovery strategy? conservationists. Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to For more information achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the To learn more about species at risk recovery in Ontario, survival and recovery of the species. It also makes please visit the Ministry of Natural Resources Species at recommendations on the objectives for protection and Risk webpage at: www.ontario.ca/speciesatrisk recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. -
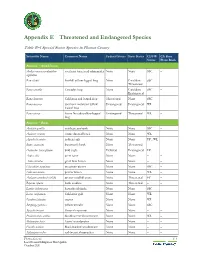
Appendix E Threatened and Endangered Species
Appendix E Threatened and Endangered Species Table E–1 Special Status Species in Plumas County Scientific Name Common Name Federal Status State Status CDFW CA Rare Status Plant Rank Animals – Amphibians Ambystoma macrodactylum southern long-toed salamander None None SSC – sigillatum Rana boylii foothill yellow-legged frog None Candidate SSC – Threatened Rana cascadae Cascades frog None Candidate SSC – Endangered Rana draytonii California red-legged frog Threatened None SSC – Rana muscosa southern mountain yellow- Endangered Endangered WL – legged frog Rana sierrae Sierra Nevada yellow-legged Endangered Threatened WL – frog Animals – Birds Accipiter gentilis northern goshawk None None SSC – Accipiter striatus sharp-shinned hawk None None WL – Aquila chrysaetos golden eagle None None FP ; WL – Buteo swainsoni Swainson's hawk None Threatened – – Haliaeetus leucocephalus bald eagle Delisted Endangered FP – Ardea alba great egret None None – – Ardea herodias great blue heron None None – – Charadrius montanus mountain plover None None SSC – Falco mexicanus prairie falcon None None WL – Antigone canadensis tabida greater sandhill crane None Threatened FP – Riparia riparia bank swallow None Threatened – – Lanius ludovicianus loggerhead shrike None None SSC – Larus californicus California gull None None WL – Pandion haliaetus osprey None None WL – Setophaga petechia yellow warbler None None SSC – Spizella breweri Brewer's sparrow None None – – Phalacrocorax auritus double-crested cormorant None None WL – Melanerpes lewis Lewis' woodpecker None -

Climate and Floristic Variation in Great Basin Mountain Ranges
David A. Charlet and Pat Leary College of Southern Nevada Funding and other support for this work: Nevada Climate Change Infrastructure for Climate Change Research, Education, and Outreach Clark County Ecosystem Indicators Project (Southern Nevada Public Land Management Act) US Geological Survey USDA Natural Resource Conservation Service US Fish and Wildlife Service Bureau of Land Management College of Southern Nevada Wesley E. Niles Herbarium, UNLV Premier location to study climate change Paleoclimate proxy data sets: Biological: fossil, pollen, woodrat midden Geomorphological: pluvial lake shores and basins, sand dunes, glacial and periglacial features Presence of all major temperate life-zones except Humid Transition Understanding our flora requires the context of climate change, isolation of populations, and refugia Hunt 1967 Plant species have specific tolerances for conditions and resource availability (e.g., temperature, nutrients, water) Vary one of the conditions (climate), and the distributions of the plants should change To detect changes in vegetation as it responds to changes in climate, we must first know where the vegetation is now We don’t know very well how species are distributed We must develop the baseline data Charlet Billings (1951) Merriam (1898) Alpine Alpine tundra MZS Arctic-Alpine Subalpine Limber pine-bristlecone pine MZS Hudsonian Montane Yellow pine-White fir MZS Canadian Pygmy Conifer Pinyon-juniper MZS Upper Sonoran Sagebrush Sagebrush-grass Upper Sonoran Blackbrush Creosote-bush Lower Sonoran -

Plant Community Composition and Structure Monitoring for Agate Fossil Beds National Monument 2011-2015 Summary Report
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Plant Community Composition and Structure Monitoring for Agate Fossil Beds National Monument 2011-2015 Summary Report Natural Resource Report NPS/NGPN/NRR—2016/1198 ON THIS PAGE Photograph of riparian long-term monitoring plot 261 at Agate Fossil Beds National Monument, 2015. Photograph courtesy of the National Park Service. ON THE COVER Photograph of plant community monitoring at Agate Fossil Beds National Monument, 2015. Photograph courtesy of the National Park Service. Plant Community Composition and Structure Monitoring for Agate Fossil Beds National Monument 2011-2015 Summary Report Natural Resource Report NPS/NGPN/NRR—2016/1198 Isabel W. Ashton Christopher J. Davis National Park Service Northern Great Plains Inventory & Monitoring Network 231 East St. Joseph Street Rapid City, SD 57701 April 2016 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. The series supports the advancement of science, informed decision-making, and the achievement of the National Park Service mission. The series also provides a forum for presenting more lengthy results that may not be accepted by publications with page limitations. -

SPRING WILDFLOWERS of OHIO Field Guide DIVISION of WILDLIFE 2 INTRODUCTION This Booklet Is Produced by the ODNR Division of Wildlife As a Free Publication
SPRING WILDFLOWERS OF OHIO field guide DIVISION OF WILDLIFE 2 INTRODUCTION This booklet is produced by the ODNR Division of Wildlife as a free publication. This booklet is not for resale. Any By Jim McCormac unauthorized reproduction is prohibited. All images within this booklet are copyrighted by the Division of Wild- life and it’s contributing artists and photographers. For additional information, please call 1-800-WILDLIFE. The Ohio Department of Natural Resources (ODNR) has a long history of promoting wildflower conservation and appreciation. ODNR’s landholdings include 21 state forests, 136 state nature preserves, 74 state parks, and 117 wildlife HOW TO USE THIS GUIDE areas. Collectively, these sites total nearly 600,000 acres Bloom Calendar Scientific Name (Scientific Name Pronunciation) Scientific Name and harbor some of the richest wildflower communities in MID MAR - MID APR Definition BLOOM: FEB MAR APR MAY JUN Ohio. In August of 1990, ODNR Division of Natural Areas and Sanguinaria canadensis (San-gwin-ar-ee-ah • can-ah-den-sis) Sanguinaria = blood, or bleeding • canadensis = of Canada Preserves (DNAP), published a wonderful publication entitled Common Name Bloodroot Ohio Wildflowers, with the tagline “Let Them Live in Your Eye Family Name POPPY FAMILY (Papaveraceae). 2 native Ohio species. DESCRIPTION: .CTIGUJQY[ƃQYGTYKVJPWOGTQWUYJKVGRGVCNU Not Die in Your Hand.” This booklet was authored by the GRJGOGTCNRGVCNUQHVGPHCNNKPIYKVJKPCFC[5KPINGNGCHGPYTCRU UVGOCVƃQYGTKPIVKOGGXGPVWCNN[GZRCPFUKPVQCNCTIGTQWPFGFNGCH YKVJNQDGFOCTIKPUCPFFGGRDCUCNUKPWU -

Wyoming FWS Endangered Species List
United States Department of the Interior FISH AND WILDLIFE SERVICE Ecological Services 5353 Yellowstone Road, Suite 308A Cheyenne, Wyoming 82009 In Reply Refer To: ES-6141 1/W.32/WY07SL0361 JAN 1 '2008 Mr. Ron C. Linton Senior Groundwater Hydrologist/Project Manager U.S. Nuclear Regulatory Commission Office of Federal and State Materials And Environmental Management Programs Mail Stop T-8F5 11545 Rockville Pike Rockville, Maryland 20852-2738 Dear Mr. Linton: Please find enclosed the U.S. Fish and Wildlife Service's (Service) current list of endangered, threatened, and candidate species which may occur within the State of Wyoming. This list is provided as a general reference for the U.S. Nuclear Regulatory Commission (NRC) to use when evaluating actions under the Endangered Species Act of 1973, as amended (Act) (16 U.S.C. 1531 et seq.). We have revised our previous species list to reflect the recovery and delisting of the bald eagle (Haliaeetusleucocephalus). On July 9, 2007, the Service published a Federal Register notice (72 FR 37346) announcing that the bald eagle would be removed from the list of threatened and endangered species under the Act on August 8, 2007. However, the protections provided to the bald eagle under the Bald and Golden Eagle Protection Act, 16 U.S.C. 668 (BGEPA) and the Migratory Bird Treaty Act, 16 U.S.C. 703 (MBTA) will remain in place. The term "disturb" under the BGEPA has recently been defined as: "to agitate or bother a bald or golden eagle to a degree that causes, or is likely to cause, based on the best scientific information available, (1) injury to an eagle, (2) a decrease in its productivity, by substantially interfering with normal breeding, feeding, or sheltering behavior, or (3) nest abandonment, by substantially interfering with normal breeding, feeding, or sheltering behavior (72 FR 31332). -

Annotated Checklist of Vascular Flora, Cedar Breaks National
National Park Service U.S. Department of the Interior Natural Resource Program Center Annotated Checklist of Vascular Flora Cedar Breaks National Monument Natural Resource Technical Report NPS/NCPN/NRTR—2009/173 ON THE COVER Peterson’s campion (Silene petersonii), Cedar Breaks National Monument, Utah. Photograph by Walter Fertig. Annotated Checklist of Vascular Flora Cedar Breaks National Monument Natural Resource Technical Report NPS/NCPN/NRTR—2009/173 Author Walter Fertig Moenave Botanical Consulting 1117 W. Grand Canyon Dr. Kanab, UT 84741 Editing and Design Alice Wondrak Biel Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 February 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientifi c community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifi cally credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. The Natural Resource Technical Report series is used to disseminate the peer-reviewed results of scientifi c studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service’s mission. The reports provide contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. Current examples of such reports include the results of research that addresses natural resource management issues; natural resource inventory and monitoring activities; resource assessment reports; scientifi c literature reviews; and peer- reviewed proceedings of technical workshops, conferences, or symposia.