Challenger's Lost Lessons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Christa Mcauliffe, Teacher Astronaut
0106C Christa McAuliffe 10/26/05 10:43 PM Page 40 Christa McAuliffe, Teacher Astronaut S ONE OF her training exercises in becoming the first teacher astronaut, A Christa McAuliffe had to curl up inside a 36-inch-diameter nylon ball. When she was zipped up, she found herself in total darkness. She didn’t know when she’d be let out. Christa wore electrodes and transmitters to see how she would react to being closed in, since on the space shuttle she’d have to share a living space that measured only 10 by 13 feet (the size of her kitchen) with six people, and she and the other astronauts would eat, sleep, go to the bathroom, work, and relax there; it was no place for someone with claustrophobia. Christa thought she would start yelling and try to claw her way out, but she lay back, folded her arms across her stomach, and imagined herself floating in space. As Christa McAuliffe a result, the 15 minutes she spent inside the nylon ball were very peaceful. At the end of the exercise, she asked if she could take the ball home. “When things start to get crazy, I can just set the timer and tell the kids, ‘O.K., Mom’s going into the sphere now.’” Christa, a high-school social studies teacher from New Hampshire, first heard about the teacher astronaut program on the radio while driving with her husband, Steve. The smile that lit Christa’s face told Steve she was interested. “Go for it,” he said. When she put off filling out the Women astronauts stand with the Personal Rescue Enclosure (the rescue ball). -

Inventory of the Ronald Mcnair Collections, Box #3
Inventory of the Ronald McNair Collections, Box #3 Contact Information Archives and Special Collections F.D. Bluford Library North Carolina A&T State University Greensboro, NC 27411 Telephone: 336-285-4176 Email: [email protected] URL: http://www.library.ncat.edu/resources/archives/ Descriptive Summary Repository F. D. Bluford Library Archives & Special Collections Creator Ronald McNair Title Ronald McNair Box #3 Language of Materials English Extent 1 archival boxes, 97 items Abstract Born Ronald Erwin McNair on October 21, 1950 in Lake City, South Carolina. In 1971, he received a bachelor of science in Physics from North Carolina A&T State University. He received a doctor of philosophy in Physics from Massachusetts Institute of Technology in 1976. He was presented with an honorary doctorate of Laws from NC A&T State University in 1978 and an honorary doctorate of Science from Morris College in 1980. He flew on a Challenger mission in February 1984. He died January 28, 1986, one of the astronauts in the Challenger disaster. The collection contains newspaper articles, recognition programs and other papers relevant to his life, both shuttle flights, and the years following the Challenger disaster in 1986. Administrative Information Restrictions to Access No Restrictions Acquisitions Information Transferred from the Office of the Chancellor. Please consult Archives Staff for additional information. Processing Information Preferred Citation [Identification of Item], Ronald McNair Box #3, Archives and Special Collections, Bluford Library, North Carolina Agricultural and Technical State University, Greensboro, NC. Copyright Notice North Carolina Agricultural and Technical College owns copyright to this collection. Individuals obtaining materials from Bluford Library are responsible for using the works in conformance with United State Copyright Law as well as any restriction accompanying the materials. -

Christa's Lost Lessons
Christa’s Lost Lessons Lost Lesson 1 Christa’s name for her mission: THE ULTIMATE FIELD TRIP (video link) Introduction: Besides the six lost science lessons scheduled for filming aboard Challenger, two televised “live lessons” were planned for the sixth day of the mission. The time scheduled for each was fifteen minutes. These were to be aired on the Public Broadcasting Network (PBS) at 10:40 a.m. and 10:40 p.m. Central Standard Time. The first lesson (actually given its name by Christa) was “The Ultimate Field Trip”. It dealt with explaining and describing to students the general layout of the shuttle. Additionally, crew members (Commander Dick Scobee, Pilot Mike Smith and others) would be introduced. The video archives contained this wonderful clip of Christa actually “walking through” a practice run of both live lessons. Christa’s Lost Lessons - Lost Lesson 1 The second live lesson is addressed in some detail in Bob Mayfield’s paper. It was entitled “Where We’ve Been, Where We’ve Going” Background: The background description for the first live lesson, “The Ultimate Field Trip”, comes from the NASA publication “Teacher in Space Project.” It is stated below: “This lesson is based on a quotation by Teacher in Space Christa McAuliffe who described her opportunity to go into space as ‘the ultimate field trip.’ Viewer Objectives: 1. To observe the major areas of the Shuttle and describe their function 2. To list and describe the major kinds of activities crewmembers perform aboard the Shuttle 3. To compare and contrast daily activities in microgravity with those on Earth. -

The Minor Planet Bulletin 44 (2017) 142
THE MINOR PLANET BULLETIN OF THE MINOR PLANETS SECTION OF THE BULLETIN ASSOCIATION OF LUNAR AND PLANETARY OBSERVERS VOLUME 44, NUMBER 2, A.D. 2017 APRIL-JUNE 87. 319 LEONA AND 341 CALIFORNIA – Lightcurves from all sessions are then composited with no TWO VERY SLOWLY ROTATING ASTEROIDS adjustment of instrumental magnitudes. A search should be made for possible tumbling behavior. This is revealed whenever Frederick Pilcher successive rotational cycles show significant variation, and Organ Mesa Observatory (G50) quantified with simultaneous 2 period software. In addition, it is 4438 Organ Mesa Loop useful to obtain a small number of all-night sessions for each Las Cruces, NM 88011 USA object near opposition to look for possible small amplitude short [email protected] period variations. Lorenzo Franco Observations to obtain the data used in this paper were made at the Balzaretto Observatory (A81) Organ Mesa Observatory with a 0.35-meter Meade LX200 GPS Rome, ITALY Schmidt-Cassegrain (SCT) and SBIG STL-1001E CCD. Exposures were 60 seconds, unguided, with a clear filter. All Petr Pravec measurements were calibrated from CMC15 r’ values to Cousins Astronomical Institute R magnitudes for solar colored field stars. Photometric Academy of Sciences of the Czech Republic measurement is with MPO Canopus software. To reduce the Fricova 1, CZ-25165 number of points on the lightcurves and make them easier to read, Ondrejov, CZECH REPUBLIC data points on all lightcurves constructed with MPO Canopus software have been binned in sets of 3 with a maximum time (Received: 2016 Dec 20) difference of 5 minutes between points in each bin. -

International Space Station Basics Components of The
National Aeronautics and Space Administration International Space Station Basics The International Space Station (ISS) is the largest orbiting can see 16 sunrises and 16 sunsets each day! During the laboratory ever built. It is an international, technological, daylight periods, temperatures reach 200 ºC, while and political achievement. The five international partners temperatures during the night periods drop to -200 ºC. include the space agencies of the United States, Canada, The view of Earth from the ISS reveals part of the planet, Russia, Europe, and Japan. not the whole planet. In fact, astronauts can see much of the North American continent when they pass over the The first parts of the ISS were sent and assembled in orbit United States. To see pictures of Earth from the ISS, visit in 1998. Since the year 2000, the ISS has had crews living http://eol.jsc.nasa.gov/sseop/clickmap/. continuously on board. Building the ISS is like living in a house while constructing it at the same time. Building and sustaining the ISS requires 80 launches on several kinds of rockets over a 12-year period. The assembly of the ISS Components of the ISS will continue through 2010, when the Space Shuttle is retired from service. The components of the ISS include shapes like canisters, spheres, triangles, beams, and wide, flat panels. The When fully complete, the ISS will weigh about 420,000 modules are shaped like canisters and spheres. These are kilograms (925,000 pounds). This is equivalent to more areas where the astronauts live and work. On Earth, car- than 330 automobiles. -

NASA Begins 5Th RS-25 Test Series
Volume 14 Issue 8 www.nasa.gov/centers/stennis August 2018 NASA begins 5th RS-25 test series NASA conducts a successful hot fire test of RS-25 developmental engine No. 0525 – featuring a new flight controller unit – on the A-1 Test Stand at Sten- nis Space Center on Aug. 14.The test was viewed by new NASA Administrator Jim Bridenstine and other guests. (See page 3 article) Page 2 LAGNIAPPE August 2018 It is estimated somewhere between 500 million to It was only fitting, then, that new NASA Administra- 600 million people around the world watched Neil tor Jim Bridenstine wasted little time in making his first Armstrong step onto the surface of the Moon in July visit to the site as agency leader. More fitting, he was 1969. It was the largest television audience at the time, able to view the Aug. 14 test and see firsthand the Sten- although they were not all in the same room. Ark! nis blended test team of NASA, Aerojet Rocketdyne and Syncom Space Services engineers and operators Probably nowhere near that many folk watched the at work. He also got a firsthand look at site facilities, NASA-TV and social media live broadcast of the RS- including the Aerojet Rocketdyne Engine Assembly 25 rocket engine test here Aug. 14 – but it is safe to say Facility, the E Test Complex and the B-2 Test Stand. an awful lot of attention is focused on Stennis Space Center these days. More importantly, the new NASA leader was able to visit with center and resident agency leaders, local Stennis is at the forefront of NASA’s work to build and media members, community representatives and site launch its new Space Launch System (SLS) rocket that employees. -

Appendix Program Managers/Acknowledgments
Flight Information Appendix Program Managers/Acknowledgments Selected Readings Acronyms Contributors’ Biographies Index Image of a Legac y—The Final Re-entry Appendix 517 Flight Information Approx. Orbiter Enterprise STS Flight No. Orbiter Crew Launch Mission Approach and Landing Test Flights and Crew Patch Name Members Date Days 1 Columbia John Young (Cdr) 4/12/1981 2 Robert Crippen (Plt) Captive-Active Flights— High-speed taxi tests that proved the Shuttle Carrier Aircraft, mated to Enterprise, could steer and brake with the Orbiter perched 2 Columbia Joe Engle (Cdr) 11/12/1981 2 on top of the airframe. These fights featured two-man crews. Richard Truly (Plt) Captive-Active Crew Test Mission Flight No. Members Date Length 1 Fred Haise (Cdr) 6/18/1977 55 min 46 s Gordon Fullerton (Plt) 2 Joseph Engle (Cdr) 6/28/1977 62 min 0 s 3 Columbia Jack Lousma (Cdr) 3/22/1982 8 Richard Truly (Plt) Gordon Fullerton (Plt) 3 Fred Haise (Cdr) 7/26/1977 59 min 53 s Gordon Fullerton (Plt) Free Flights— Flights during which Enterprise separated from the Shuttle Carrier Aircraft and landed at the hands of a two-man crew. 4 Columbia Thomas Mattingly (Cdr) 6/27/1982 7 Free Flight No. Crew Test Mission Henry Hartsfield (Plt) Members Date Length 1 Fred Haise (Cdr) 8/12/1977 5 min 21 s Gordon Fullerton (Plt) 5 Columbia Vance Brand (Cdr) 11/11/1982 5 2 Joseph Engle (Cdr) 9/13/1977 5 min 28 s Robert Overmyer (Plt) Richard Truly (Plt) William Lenoir (MS) 3 Fred Haise (Cdr) 9/23/1977 5 min 34 s Joseph Allen (MS) Gordon Fullerton (Plt) 4 Joseph Engle (Cdr) 10/12/1977 2 min 34 s Richard Truly (Plt) 5 Fred Haise (Cdr) 10/26/1977 2 min 1 s 6 Challenger Paul Weitz (Cdr) 4/4/1983 5 Gordon Fullerton (Plt) Karol Bobko (Plt) Story Musgrave (MS) Donald Peterson (MS) The Space Shuttle Numbering System The first nine Space Shuttle flights were numbered in sequence from STS -1 to STS-9. -

Space Reporter's Handbook Mission Supplement
CBS News Space Reporter's Handbook - Mission Supplement! Page 1 The CBS News Space Reporter's Handbook Mission Supplement Shuttle Mission STS-124: Space Station Assembly Flight 1J Written and Edited By William G. Harwood Aerospace Writer/Consultant [email protected] CBS News!!! 7/4/11 Page 2 ! CBS News Space Reporter's Handbook - Mission Supplement Revision History Editor's Note Mission-specific sections of the Space Reporter's Handbook are posted as flight data becomes available. Readers should check the CBS News "Space Place" web site in the weeks before a launch to download the latest edition: http://www.cbsnews.com/network/news/space/current.html DATE RELEASE NOTES 05/28/08 Initial STS-124 release Introduction This document is an outgrowth of my original UPI Space Reporter's Handbook, prepared prior to STS-26 for United Press International and updated for several flights thereafter due to popular demand. The current version is prepared for CBS News. As with the original, the goal here is to provide useful information on U.S. and Russian space flights so reporters and producers will not be forced to rely on government or industry public affairs officers at times when it might be difficult to get timely responses. All of these data are available elsewhere, of course, but not necessarily in one place. The STS-124 version of the CBS News Space Reporter's Handbook was compiled from NASA news releases, JSC flight plans, the Shuttle Flight Data and In-Flight Anomaly List, NASA Public Affairs and the Flight Dynamics office (abort boundaries) at the Johnson Space Center in Houston. -

Acronyms, Abbreviations, and Glossary
APPENDIX 1 Acronyms, Abbreviations, and Glossary AA Associate Administrator AAA Active Acquisition Aid AAP Apollo Applications Program ACS attitude control system ACN Ascension ACU antenna control unit AES Apollo Extension System AFSC Air Force Systems Command AFSCN Air Force Satellite Control Network AGO Santiago AIS Apollo Instrumentation Ship ALT Approach and Landing Test CDR Critical Design Review CDSCC Canberra Deep Space Communication Complex CSIR Council for Scientific and Industrial Research CSIRO Commonwealth Scientific and Industrial Research Organization CSM Command and Service Module CSOC Consolidated Space Operations Contract DAF Data Acquisition Facility dB decibels DDMS Department of Defense Manager for Manned Spaceflight 404 “Read You Loud and Clear!” DF direction finding DJS Dzhusaly, Razakhgtan DLR Germany’s Deutsches Zentrum für Luftund Raumfahrt DOD Department of Defense DOI Department of the Interior DOS Department of Supply DRSS Data Relay Satellite System DSN Deep Space Network DSS Deep Space Station EGO Eccentric Geophysical Observatory EGR Eglin Gulf Test Range ELVIS Enhanced Launch Vehicle Imaging System ERS Earth Resource Satellite ERTS Earth Resource Technology Satellite ESA European Space Agency ESD Air Force Electronic Systems Division ESMC Air Force Eastern Space and Missile Center EUMETSAT European Organization for the Exploitation of Meteorological Satellites ETR Eastern Test Range EVA extravehicular activity EUT Eupatona, Ukraine FAA Federal Aviation Administration FCC Federal Communications Commission -

Challenger Disaster
Challenger Disaster 0. Challenger Disaster - Story Preface 1. EARLY WARNINGS 2. AN ACCIDENT ROOTED IN HISTORY 3. WARNINGS IGNORED 4. LAST-MINUTE PLEAS 5. THE FINAL MINUTES 6. THE EXPLOSION 7. THE LAST WORDS 8. THE HORROR OF DESTRUCTION 9. THE UNTHINKABLE 10. THE TRANSCRIPTS 11. CHALLENGER'S AFTERMATH Space Shuttle landing. Online courtesy NASA They slipped the surly bonds of earth To touch the face of God. President Reagan January 28, 1986 It was the 25th time a U.S. Space Transportation System would take off from Cape Canaveral. This was no ordinary flight, however. Christa McAuliffe was America’s first teacher to fly in space. With her training complete, and school children anticipating her trip, there was a sense of excitement throughout the country on the evening of January 27, 1986. People were so used to the flights, most folks no longer watched shuttle liftoffs. This flight was different, though. Everyone - including school children - would be watching because Christa McAuliffe was going into space. But the shuttle’s launch mechanism had a fatal design flaw. As a result, Challenger and her seven-member crew - including America’s first teacher in space - were blown out of the sky seventy-three seconds after launch on January 28, 1986. See Alignments to State and Common Core standards for this story online at: http://www.awesomestories.com/asset/AcademicAlignment/Challenger-Disaster See Learning Tasks for this story online at: http://www.awesomestories.com/asset/AcademicActivities/Challenger-Disaster Media Stream Supersonic Transportation System Image online, courtesy NASA. Photo of the space shuttle "Discovery," taken on September 12, 1993, during the launch of Mission STS-51. -
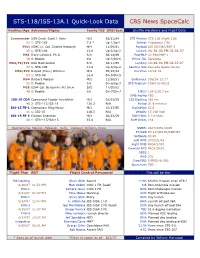
STS-118/ISS-13A.1 Quick-Look Data CBS News Spacecalc
STS-118/ISS-13A.1 Quick-Look Data CBS News SpaceCalc Position/Age Astronaut/Flights Family/TIS DOB/Seat Shuttle Hardware and Flight Data Commander USN Cmdr. Scott J. Kelly M/2 02/21/64 STS Mission STS-118 (flight 119) 43 1: STS-103 7.9 * Up-1/Up-1 Orbiter Endeavour (19) Pilot USMC Lt. Col. Charles Hobaugh M/4 11/05/61 Payload ISS S5/CMG/ESP-3 45 1: STS-104 12.8 Up-2/Up-2 Launch 06:36:36 PM 08.08.07 MS1 Tracy Caldwell, Ph.D. S/0 08/14/69 Pad/MLP LC-39A/MLP-1 38 0: Rookie 0.0 Up-3/Dn-6 Prime TAL Zaragoza MS2/FE/EV1 Rick Mastracchio S/0 02/11/60 Landing 12:49:00 PM 08.22.07 47 1: STS-106 11.8 Up-4/Up-4 Landing Site Kennedy Space Center MS3/EV2 Dafydd (Dave) Williams M/2 05/16/54 Duration 13/18:12 53 1: STS-90 16.0 Dn-5/Dn-5 MS4 Barbara Morgan M/2 11/28/51 Endeavour 206/14:12:17 55 0: Rookie 0.0 Dn-6/Up-3 STS Program 1096/16:48:21 MS5 USAF Col. Benjamin (Al) Drew S/0 11/05/62 48 0: Rookie 0.0 Dn-7/Dn-7 MECO 134.8/35.7 sm OMS Ha/Hp TBD ISS-15 CDR Cosmonaut Fyodor Yurchikhin M/2 01/03/59 ISS Docking 220 sm 48 2: STS-112,ISS-15 129.3 N/A Period 91.6 minutes ISS-15 FE-1 Cosmonaut Oleg Kotov M/2 10/27/65 Inclination 51.6 41 1: ISS-15 118.3 N/A Velocity 17,188 mph ISS-15 FE-2 Clayton Anderson M/2 02/23/59 EOM Miles 5.7 million 48 1: STS-117/ISS-15 55.8 N/A EOM Orbits 218 Mastrachchio, Morgan, Hobaugh, Kelly, Caldwell, Williams, Drew SSMEs 2047/2051/2045 ET/SRB ET-117/Bi130/RSRM97 Software OI-30 Left OMS LP03/31/F1 Right OMS RP04/27/F1 Forward RCS FRC5/20/F1 OBSS 1 RMS 201 Cryo/GN2 5 PRSD/6 GN2 Spacesuits TBD Flight Plan EDT Flight Control Personnel This will be the… ISS Docking Steve Stich Ascent 119th Shuttle mission since STS-1 8/10/07 01:51 PM Matt Abbott Orbit 1 FD (lead) 6th Post-Columbia mission EVA-1 Richard Jones Orbit 2 FD 94th Post-Challenger mission 8/11/07 01:07 PM Mike Moses Planning 20th Flight of Endeavour EVA-2 Steve Stich Entry 90th Day launch 8/13/07 12:07 PM K. -

AUTHOR Engle, Harry A.; Christensen, David L. TITLE Educational Planning for Utilization of Space Shuttle (ED-PLUSS)
DOCUMENT RESUME ED 104 429 IR 001 833 AUTHOR Engle, Harry A.; Christensen, David L. TITLE Educational Planning for Utilization of Space Shuttle (ED-PLUSS). Final Research Report. INSTITUTION Alabama Univ., Huntsville. School of Graduate Studies and Research. SPONS AGENCY National Aeronautics and Space Administration, Huntsville, Ala. George C. Marshall Space Flight Center. PUB DATE Sep 74 NOTE 132p. EDRS PRICE MF-80.76HC-86.97 PLUS POSTAGE DESCRIPTORS *Aerospace Technology; Communication Satellites; Cost Effectiveness; Educational Experiments; Educational Innovation; * Educational Planning; Educational Research; *Educational Technology; Facility Utilization Research; Feasibility Studies; Information Utilization; Instructional Media IDENTIFIERS ED PLUSS; National Aeronautics and Space: Administration; *Skylab Student Experiment Program; Space Shuttle; Space Transportation System ABSTRACT Possible educational uses of the proposed space-shuttle program of the National Aeronautics and Space Administration are outlined. Potential users of information developed by the project are identified and their characteristicsanalyzed. Other space-education programs operated by NASA are detailed. Proposals for a methodology for expanding educational use are offered, along with methods which might be employed to increase viewer awareness, including educational networkconsiderations. (SK) IDENTIFICATION AND EVALUATION OF EDUCATIONAL USES AND USERS FOR THE STS Working Title For This Study EDUCATIONAL PLANNING FORUTILIZATION OF SPACE SHUTTLE (ED-PLUSS) By H. A. Engle and D. L. Christensen Final Research Report This research work was supported by the National Aeronautics and Space Administration George C. Marshall Space Flight Center Contract NAS8-30737 The University Of Alabama In Huntsville September 1974 IDENTIFICATION AND EVALUATION OFEDUCATIONAL USES AND USERS FOR THE STS Working Title For This Study EDUCATIONAL PLANNING FORUTILIZATION OF SPACE SHUTTLE ED-PLUSS By Harry A.