Avil® 25 Avil®50
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Opioid and Other Substance Use Disorders
OPIOID AND OTHER SUBSTANCE USE DISODERS Dr Amit Arya Additional Professor Department of Psychiatry KGMU Lucknow What are addictive substances? Any substance which when taken has an ability to change the person’s consciousness, thinking, mood, behaviour and motor functions Leading to take the substance repeatedly (World Health Organisation, 1992) …Also called as psychoactive substances Layman term: “Drugs” Why are certain substances addictive? Intake of any substance – oral, inhalational, injecting Enters the bloodstream Acts on a specific body part, such as heart, lung, stomach, etc. Addictive substances act on brain Addictive Substances act on brain All substances acting on the brain are not addictive Addictive substances I want to primarily act on a particular take that area/group of neurons in the drug again! brain, Leading the individual to repeatedly administer the addictive / psychoactive substance → “drug seeking” behaviour Addictive substances primarily act on a particular area/group of neurons in the brain. Regions controlling emotions, thinking, Frontal region judgement & memory Mid Brain How are addictive substances different from each other? Broad actions Chemical that the drug class of drugs produces on the brain Source of drug Natural/semi- TYPOLOGY synthetic/synthetic Mode of intake Oral/inhalational/ Availability – parenteral legal/illegal? (injections) Typology – Chemical Class Opioids Alcohol Cannabis Volatile solvents Based on chemical class Stimulants Tobacco Sedative- hypnotics Hallucinogens The usual drug-use -

Antihistamine Therapy in Allergic Rhinitis
CLINICAL REVIEW Antihistamine Therapy in Allergic Rhinitis Paul R. Tarnasky, MD, and Paul P. Van Arsdel, Jr, MD Seattle, Washington Allergic rhinitis is a common disorder that is associated with a high incidence of mor bidity and considerable costs. The symptoms of allergic rhinitis are primarily depen dent upon the tissue effects of histamine. Antihistamines are the mainstay of therapy for allergic rhinitis. Recently, a second generation of antihistamines has become available. These agents lack the adverse effect of sedation, which is commonly associated with older antihistamines. Current practice of antihistamine therapy in allergic rhinitis often involves random selection among the various agents. Based upon the available clinical trials, chlorpheniramine appears to be the most reasonable initial antihistaminic agent. A nonsedating antihis tamine should be used initially if a patient is involved in activities where drowsiness is dangerous. In this comprehensive review of allergic rhinitis and its treatment, the cur rent as well as future options in antihistamine pharmacotherapy are emphasized. J Fam Pract 1990; 30:71-80. llergic rhinitis is a common condition afflicting some defined by the period of exposure to those agents to which A where between 15 and 30 million people in the United a patient is sensitive. Allergens in seasonal allergic rhinitis States.1-3 The prevalence of disease among adolescents is consist of pollens from nonflowering plants such as trees, estimated to be 20% to 30%. Two thirds of the adult grasses, and weeds. These pollens generally create symp allergic rhinitis patients are under 30 years of age.4-6 Con toms in early spring, late spring through early summer, sequently, considerable costs are incurred in days lost and fall, respectively. -
![With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S](https://docslib.b-cdn.net/cover/2862/with-3h-mepyramine-trieyclic-antidepressants-antihistamine-neurotransmitter-amitriptyline-vinh-tan-tran-raymond-s-1512862.webp)
With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S
Proc. Nati. Acad. Sci. USA Vol. 75, No. 12, pp. 6290-6294,, December 1978 Neurobiology Histamine H1 receptors identified in mammalian brain membranes with [3H]mepyramine (trieyclic antidepressants/antihistamine/neurotransmitter/amitriptyline) VINH TAN TRAN, RAYMOND S. L. CHANG, AND SOLOMON H. SNYDER* Departments of Pharmacology and Experimental Therapeutics, and Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205 Communicated by Julius Axelrod, August 30,1978 ABSTRACT The antihistamine [3H mepyramine binds to Male Sprague-Dawley rats (150-200 g) were killed by cer- HI histamine receptors in mammalian brain membranes. vical dislocation, their brains were rapidly removed and ho- Potencies of H1 antihistamines at the binding sites correlate mogenized with a Polytron for 30 min (setting 5) in 30 vol of with their pharmacological antihistamine effects in the guinea pig ileum. Specific [3Himepyramine binding is saturable with ice-cold Na/K phosphate buffer (50 mM, pH 7.5), and the a dissociation constant of about 4 nM in both equilibrium and suspension was centrifuged (50,000 X g for 10 min). The pellet kinetic experiments and a density of 10pmolper gram ofwhole was resuspended in the same volume of fresh buffer and cen- brain. Some tricyclic antidepressants are potent inhibitors of trifuged, and the final pellet was resuspended in the original secific [3Hmepamine binding. Regional variations of volume of ice-cold buffer by Polytron homogenization. Calf [3Hjmepyramine ing do not correlate with variations in brains were obtained from a local abattoir within 2 hr after the endogeneous histamine and histidine decarboxylase activity. death of the animals and transferred to the laboratory in ice- Histamine is a neurotransmitter candidate in mammalian brain cold saline. -

Some Pharmacological Properties of Trimethoxy Analogs of Pheniramine, Tripelennamine, and Diphenhydramine
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 1966 Some Pharmacological Properties of Trimethoxy Analogs of Pheniramine, Tripelennamine, and Diphenhydramine Ronald Otto Langner University of Rhode Island Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Langner, Ronald Otto, "Some Pharmacological Properties of Trimethoxy Analogs of Pheniramine, Tripelennamine, and Diphenhydramine" (1966). Open Access Master's Theses. Paper 200. https://digitalcommons.uri.edu/theses/200 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. SOME PHARMACOLOGICAL PRDPERTIES OF TRIMETHOXY ANALOGS OF PHENIRAMINE,_ TRIPELENNAMINE, AND DIPHENHYDRAMINE BY RONALD OT'ID LANGNER A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FDR THE DEGREE OF MASTER OF SCIENCE IN PHARMACOLOGY UNIVERSITY OF RHODE ISLAND 1966 ABSTRACT Trimethoxy analogs of tripelennamine, diphenhydramine, and pheniramine were studied to determine the influences of the tri methoxy group on the pharmacological activity of known antihista mines. In this investigation, the ability of the parent compounds and their analogs to antagonize the histamine-induced contractions of guinea pig ilia were studied as well as their effects on the systolic blood pressure of male albino rats and on the central nervous system of mice as measured by the actophotometer. 'lhe trimethoxy analogs were comparatively weak competitive antagonists of histamine with the exception of N,N,-Diethyl-N' (2-pyridyl)-N'-(3,4,S-trimethoxybenzyl) ethylenediamine Dicyclamate (De-TMPBZ) and N,N,-Diethyl-N'-(2-pyridyl)-N'-(3,4,5-trimethoxy benzyl) ethylenediamine Disuccinate (TMPBZ) which were weak non competitive histamine antagonists. -
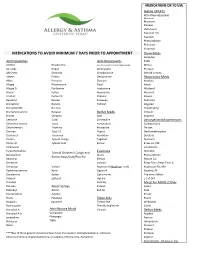
Medications to Avoid Minimum 7 Days Prior to Appointment
MEDICATIONS OK TO USE NASAL SPRAYS Afrin (Oxymetazoline) Atrovent Beconase Flonase Fluticasone Nasocort AQ Nasonex Phenylephrine Rhinocort Veramyst MEDICATIONS TO AVOID MINIMUM 7 DAYS PRIOR TO APPOINTMENT Derm Meds Atopiclair Antihistamines Anti-Depressants Elidel Actifed Novahistine (only at the advice of the prescribing physician) Mimyx Aerokids Nyquil Amitripyline Protopic AH-Chew Omnihist Clomipramine Steroid Creams Alavert Pediox Desipramine Respiratory Meds Aldex Periactin Doxepin Accolate Allegra Pheniramine Elavil Advair Allegra D Pyrilamine Imipramine Albuterol Atarax Pyrlex Norpramin Atrovent Atrohist Pyrlex CB Pamelor Flovent Benadryl Rescon Sinequan Pulmicort Bromphed Rondec Tofranil Singulair Bromphed PD Ru-Tuss Theophylline Brompheniramine Rynatan Reflux Meds Ventolin Brovex Semprex Axid Xopenex Cetirizine Sudal Cimetadine Decongestants/Expectorants Chlorpheniramine Tavist Famotadine Carbapentane Chlortrimeton Triaminic Nizatadine Delsym Clarinex Tussi-12 Pepcid Dextromethorphan Clarinex D Tussionex Ranitidine Duratuss Claritin Tylenol Allergy Tagamet Duravent Claritin D Tylenol Cold Zantac Entex LA, PSE Clemastine Guaifenisen Cyrproheptadine Humabid Tylenol Children's Cough and Eyedrops Deconamine Alocril Phenylephrine Runny Nose/Cold/Plus Flu Dexodryl Elestat Rescon GG Dimetane Livostin Respi-Tann, Respi-Tann G Dimetapp Vistaril Naphcon-A (Naphcon is ok) Robitussin PD, DM Diphenhydramine Xyzal Opcon-A Sudafed, PE Doxylamine Zyrtec Optichrome Triaminic Yellow Drixoral Zyrtec D Optivar Z-Cof DM Duradryl Pataday Meds for -

AVIL® Pheniramine Maleate Tablets & Injection
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory AVIL® Pheniramine Maleate Tablets & Injection THERAPEUTIC CATEGORY Antihistaminic COMPOSITION AVIL 25 Each uncoated tablet contains: Pheniramine Maleate IP…25mg AVIL 50 Each uncoated tablet contains: Pheniramine Maleate IP…50mg AVIL Injection (in 2ml ampoule) Each ml contains: Pheniramine Maleate IP …22.75mg AVIL Injection (in 10ml vial) Each ml contains: Pheniramine Maleate IP …… 22.75mg Methyl-Parahydroxybenzoate IP ……0.135%w/v Propyl-Parahydroxybenzoate IP ……0.015%w/v Water for Injection ………… q.s THERAPEUTIC INDICATIONS Allergic conditions (Hay fever, drug rashes, angioneurotic oedema, serum sickness, allergic conjunctivitis, food allergy etc.), Conditions of the respiratory tract that are accompanied by increased secretion, All itching skin conditions, Prevention and treatment of motion sickness, nausea, vomiting and vertigo due to Menière’s disease and other labyrinthine disturbances. DOSAGE AND ADMINISTRATION The dosage should be adjusted individually. When immediate or rapid response is desired, intravenous or intramuscular Avil injection should be used. Tablets: Adults & Children (above 10 years): Initially 1 tablet of Avil 25 two to three times a day. The dose may be increased to two tablets of Avil 25 or one tablet of Avil 50, administered two to three times a day if needed. The maximum dose of 3mg/kg/day should not be exceeded. Children 5-10 years of age: Half a tablet up to three times daily. Avil® tablets are not recommended in children under 5 years of age. Do not take the medicine on an empty stomach. For travel sickness, the dose should be taken atleast 30 mins before travelling. -

Autacoids Introduction
AUTACOIDS INTRODUCTION • AUTACOIDS auto=self akos=healing/remedy • Local Hormones CLASSIFICATION • Amine derived: Histamine (amino acid: Histidine), Serotonin (Tryptophan) • Peptide derived: Angiotensin, Bradykinin • Lipid derived: Prostaglandins, Leukotrienes, Interleukins, Platelet Activating Factor, etc. FUNCTIONS • Physiological • Pathophysiological (Reaction to injuries) • Transmission and Modulation AMINE AUTACOIDS • DERIVED FROM NATURAL AMINO ACIDS • HISTAMINE AND SEROTONIN are the major autacoids in this class HISTAMINE NH2 5 4 1 3 N N H 2 Histamine INTRODUCTION • Imidazole ethylamine • Formed from the amino acid Histidine • Important inflammatory mediator • Potent biogenic amine and plays an important role in inflammation, anaphylaxis, allergies, gastric acid secretion and drug reaction • As part of an immune response to foreign pathogens, its produced by Basophils and mast cells found in nearby connective tissues. SITES OF HISTAMINE RELEASE 1) Mast cell site: Pulmonary tissue (mucosa of bronchial tree) • Skin • GIT(intestinal mucosa) • Conc. Of histamine is particularly high in these tissues 2) Non-mast cell sites: • CNS (neurons) • Epidermis of skin. • GIT(gastric cells) • Cells in regenerating or rapidly growing tissues • Basophils (in the blood) MECHANISM OF RELEASE • Histamine held by an acidic protein and heparin within intracellular granules → Granules extrude by exocytosis → Na+ gets exchanged for histamine • Substances released during IgG or lgM immunoreactions release histamine from the mast cells & basophil. • Chemical -

NIH Public Access Author Manuscript Birth Defects Res a Clin Mol Teratol
NIH Public Access Author Manuscript Birth Defects Res A Clin Mol Teratol. Author manuscript; available in PMC 2013 April 08. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Birth Defects Res A Clin Mol Teratol. 2009 February ; 85(2): 137–150. doi:10.1002/bdra.20513. Use of Antihistamine Medications During Early Pregnancy and Isolated Major Malformations Suzanne M. Gilboa*, Matthew J. Strickland*, Andrew F. Olshan†, Martha M. Werler‡, Adolfo Correa*, and The National Birth Defects Prevention Study *National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, GA †Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC ‡Slone Epidemiology Center at Boston University, Boston, MA Abstract BACKGROUND—Antihistamines are commonly used during pregnancy. There is little evidence that they have teratogenic effects, but there are knowledge gaps with respect to newer products, as well as the relationship between specific antihistamines and specific birth defects. METHODS—Using the National Birth Defects Prevention Study (1997-2003), the authors examined associations between maternal use of 14 antihistamines during early pregnancy and 26 isolated major birth defects. A Bayesian analysis incorporating prior knowledge about the relationships between the antihistamines, birth defects, and measured covariates was conducted. RESULTS—Of the 364 associations investigated, 24 had 95% posterior intervals excluding 1.0. All 24 associations were positive; 23 associations were of weak to moderate magnitude (posterior odds ratio [OR] < 3.0) and one was strong (OR > 6.0) but very imprecise. Of the 24 associations, 20 were with non-cardiac defects. -

Identify Diagnostics Drug Test Cups Dip Card FUO INSERT 2 2019
failure, and even death. Many cases of overdose are caused by patients inadvertently taking The oral bioavailability of gabapentin is approximately 80% at 100 mg but decreases to 60% at Drug Tests (Strip/Card/Device/Cup) more than the recommended dose (i.e., 4 grams a day) of a particular product, or by taking more 300 mg, 47% at 400 mg, 34% at 800 mg, 33% at 1,200 mg, and 27% at 1,600 mg. Package Insert for testing of any combination of the following drugs: than one product containing acetaminophen (e.g., an OTC product and an Rx drug containing Drugs that increase the transit time of gabapentin in the small intestine can increase its oral APAP/COT/CLZP/EDDP/ETG/FEN/GBPT/KET/K2/K3/LSD/MDPV/MQL/MTHP/TRA/ZOLP/6-M acetaminophen). The mechanism of liver injury is not related to acetaminophen itself, but to the bioavailability; when gabapentin was co-administered with oral morphine (which slows AM production of a toxic metabolite. The toxic metabolite binds with liver proteins, which cause intestinal peristalsis),the oral bioavailability of a 600 mg dose of gabapentin increased by 50%. Available with Specimen Validity Tests (S.V.T.) for: cellular injury. The ability of the liver to remove this metabolite before it binds to liver protein Gabapentin at a low dose of 100 mg has a Tmax (time to peak levels) of approximately 1.7 hours, Oxidants/PCC, Specific Gravity, pH, Nitrite, Glutaraldehyde and Creatinine influences the extent of liver injury. while the Tmax increases to 3 to 4 hours at higher doses. -

NIH Public Access Author Manuscript Bioorg Med Chem
NIH Public Access Author Manuscript Bioorg Med Chem. Author manuscript; available in PMC 2011 May 5. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Bioorg Med Chem. 2009 September 15; 17(18): 6496±6504. doi:10.1016/j.bmc.2009.08.016. Synthesis, Structure-Affinity Relationships and Modeling of AMDA Analogs at 5-HT2A and H1 Receptors: Structural Factors Contributing to Selectivity Jitesh R. Shaha, Philip D. Mosiera, Bryan L. Rothb, Glen E. Kellogga, and Richard B. Westkaempera,* a Department of Medicinal Chemistry, School of Pharmacy, Virginia Commonwealth University, Richmond, VA 23298 USA b Department of Pharmacology, University of North Carolina School of Medicine, Chapel Hill, NC 27599 USA Abstract Histamine H1 and serotonin 5-HT2A receptors present in the CNS have been implicated in various neuropsychiatric disorders. 9-Aminomethyl-9,10-dihydroanthracene (AMDA), a conformationally constrained diarylalkyl amine derivative, has affinity for both of these receptors. A structure- affinity relationship (SAFIR) study was carried out studying the effects of N-methylation, varying the linker chain length and constraint of the aromatic rings on the binding affinities of the compounds with the 5-HT2A and H1 receptors. Homology modeling of the 5-HT2A and H1 receptors suggests that AMDA and its analogs, the parent of which is a 5-HT2A antagonist, can bind in a fashion analogous to that of classical H1 antagonists whose ring systems are oriented towards the fifth and sixth transmembrane helices. The modeled orientation of the ligands are consistent with the reported site-directed mutagenesis data for 5-HT2A and H1 receptors and provide a potential explanation for the selectivity of ligands acting at both receptors. -

Pheniramine Hydrogen Maleate Dependence: a Case Report
PHENIRAMINE HYDROGEN MALEATE DEPENDENCE: A CASE REPORT a* Mehmet Hamdi ÖRÜM a Specialist, Psychiatry, Kahta State Hospital, Adiyaman, Turkey. ARTICLE INFO ABSTRACT Article history: Pheniramine hydrogen maleate (PHM) is histamine antagonist, used for the Received: 12 July 2019 treatment of allergic disorders. PHM can cause side effects such as seda- Accepted: 12 February 2019 tion and euphoria and with the potential for tolerance to the sedative effect Available Online: 10 September 2020 of PHM and physical withdrawal symptoms refer to PHM abuse. The pa- tients who abuse PHM were interested in its features such as hallucinogen, Key Words: inexpensive, easily and legally available. Healthcare professionals also ap- Addiction, pheniramine hydrogen maleate, ply the drug easily and in a riskier group. Additional history of psychiatric psychometric scale, substance use disorder disorder or personality pattern contributes to addiction potential. Misinter- pretation of hallucinatory behaviours can lead to a vicious cycle of depend- *Correspondence: Mehmet Hamdi Orum ence. Herein, we presented a 30-year-old male patient with history of use of Address: Psychiatry, Kahta State Hospital Adiyaman — Turkey, 02100 high doses of PHM and discussed the treatment process. Tel: +90 416 216 10 15/1186 E-mail: [email protected] Turkish Journal of Health Science and Life 2020, Vol.3, No.1, 15-18 1. Introduction effect of PHM and physical withdrawal symptoms refer to PHM abuse (3, 4). Herein, we discussed a Pheniramine hydrogen maleate (PHM) is an alkyl 30-year-old male patient with history of use of high amine-derived histamine 1 (H1) antagonist, used doses of PHM. for the treatment of rhinitis, allergic dermatoses, and pruritus (1, 2). -

Histamine, Histamine Receptors, and Their Role in Immunomodulation: an Updated Systematic Review
The Open Immunology Journal, 2009, 2, 9-41 9 Open Access Histamine, Histamine Receptors, and their Role in Immunomodulation: An Updated Systematic Review Mohammad Shahid*,1, Trivendra Tripathi2, Farrukh Sobia1, Shagufta Moin2, Mashiatullah Siddiqui2 and Rahat Ali Khan3 1Section of Immunology and Molecular Biology, Department of Microbiology, 2Department of Biochemistry, and 3Department of Pharmacology, Faculty of Medicine, Jawaharlal Nehru Medical College & Hospital, Aligarh Muslim University, Aligarh-202002, U.P., India Abstract: Histamine, a biological amine, is considered as a principle mediator of many pathological processes regulating several essential events in allergies and autoimmune diseases. It stimulates different biological activities through differen- tial expression of four types of histamine receptors (H1R, H2R, H3R and H4R) on secretion by effector cells (mast cells and basophils) through various immunological or non-immunological stimuli. Since H4R has been discovered very re- cently and there is paucity of comprehensive literature covering new histamine receptors, their antagonists/agonists, and role in immune regulation and immunomodulation, we tried to update the current aspects and fill the gap in existing litera- ture. This review will highlight the biological and pharmacological characterization of histamine, histamine receptors, their antagonists/agonists, and implications in immune regulation and immunomodulation. Keywords: Histamine, histamine receptors, H4-receptor, antagonists, agonists, immunomodulation. I.