Some Pharmacological Properties of Trimethoxy Analogs of Pheniramine, Tripelennamine, and Diphenhydramine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Opioid and Other Substance Use Disorders
OPIOID AND OTHER SUBSTANCE USE DISODERS Dr Amit Arya Additional Professor Department of Psychiatry KGMU Lucknow What are addictive substances? Any substance which when taken has an ability to change the person’s consciousness, thinking, mood, behaviour and motor functions Leading to take the substance repeatedly (World Health Organisation, 1992) …Also called as psychoactive substances Layman term: “Drugs” Why are certain substances addictive? Intake of any substance – oral, inhalational, injecting Enters the bloodstream Acts on a specific body part, such as heart, lung, stomach, etc. Addictive substances act on brain Addictive Substances act on brain All substances acting on the brain are not addictive Addictive substances I want to primarily act on a particular take that area/group of neurons in the drug again! brain, Leading the individual to repeatedly administer the addictive / psychoactive substance → “drug seeking” behaviour Addictive substances primarily act on a particular area/group of neurons in the brain. Regions controlling emotions, thinking, Frontal region judgement & memory Mid Brain How are addictive substances different from each other? Broad actions Chemical that the drug class of drugs produces on the brain Source of drug Natural/semi- TYPOLOGY synthetic/synthetic Mode of intake Oral/inhalational/ Availability – parenteral legal/illegal? (injections) Typology – Chemical Class Opioids Alcohol Cannabis Volatile solvents Based on chemical class Stimulants Tobacco Sedative- hypnotics Hallucinogens The usual drug-use -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Questions in the Chemical Enzymology of MAO
Review Questions in the Chemical Enzymology of MAO Rona R. Ramsay 1,* and Alen Albreht 2 1 Biomedical Sciences Research Complex, School of Biology, University of St Andrews, St Andrews KY16 9ST, UK 2 Laboratory for Food Chemistry, Department of Analytical Chemistry, National Institute of Chemistry, Hajdrihova 19, SI-1000 Ljubljana, Slovenia; [email protected] * Correspondence: [email protected]; Tel.: +44-(0)-1334-474740 Abstract: We have structure, a wealth of kinetic data, thousands of chemical ligands and clinical information for the effects of a range of drugs on monoamine oxidase activity in vivo. We have comparative information from various species and mutations on kinetics and effects of inhibition. Nevertheless, there are what seem like simple questions still to be answered. This article presents a brief summary of existing experimental evidence the background and poses questions that remain intriguing for chemists and biochemists researching the chemical enzymology of and drug design for monoamine oxidases (FAD-containing EC 4.1.3.4). Keywords: chemical mechanism; kinetic mechanism; oxidation; protein flexibility; cysteine modifica- tion; reversible/irreversible inhibition; molecular dynamics; simulation 1. Introduction Monoamine oxidase (E.C. 1.4.3.4) enzymes MAO A and MAO B are FAD-containing Citation: Ramsay, R.R.; Albreht, A. proteins located on the outer face of the mitochondrial inner membrane, retained there Questions in the Chemical Enzymology of MAO. Chemistry 2021, by hydrophobic interactions and a transmembrane helix. The redox co-factor (FAD) is 3, 959–978. https://doi.org/10.3390/ covalently attached to a cysteine and buried deep inside the protein [1]. -

Azilect, INN-Rasagiline
SCIENTIFIC DISCUSSION 1. Introduction AZILECT is indicated for the treatment of idiopathic Parkinson’s disease (PD) as monotherapy (without levodopa) or as adjunct therapy (with levodopa) in patients with end of dose fluctuations. Rasagiline is administered orally, at a dose of 1 mg once daily with or without levodopa. Parkinson’s disease is a common neurodegenerative disorder typified by loss of dopaminergic neurones from the basal ganglia, and by a characteristic clinical syndrome with cardinal physical signs of resting tremor, bradikinesia and rigidity. The main treatment aims at alleviating symptoms through a balance of anti-cholinergic and dopaminergic drugs. Parkinson’s disease (PD) treatment is complex due to the progressive nature of the disease, and the array of motor and non-motor features combined with early and late side effects associated with therapeutic interventions. Rasagiline is a chemical inhibitor of the enzyme monoamine oxidase (MAO) type B which has a major role in the inactivation of biogenic and diet-derived amines in the central nervous system. MAO has two isozymes (types A and B) and type B is responsible for metabolising dopamine in the central nervous system; as dopamine deficiency is the main contributing factor to the clinical manifestations of Parkinson’s disease, inhibition of MAO-B should tend to restore dopamine levels towards normal values and this improve the condition. Rasagiline was developed for the symptomatic treatment of Parkinson’s disease both as monotherapy in early disease and as adjunct therapy to levodopa + aminoacids decarboxylase inhibitor (LD + ADI) in patients with motor fluctuations. 2. Quality Introduction Drug Substance • Composition AZILECT contains rasagiline mesylate as the active substance. -

Evidence That Formulations of the Selective MAO-B Inhibitor, Selegiline, Which Bypass First-Pass Metabolism, Also Inhibit MAO-A in the Human Brain
Neuropsychopharmacology (2015) 40, 650–657 OPEN & 2015 American College of Neuropsychopharmacology. All rights reserved 0893-133X/15 www.neuropsychopharmacology.org Evidence that Formulations of the Selective MAO-B Inhibitor, Selegiline, which Bypass First-Pass Metabolism, also Inhibit MAO-A in the Human Brain Joanna S Fowler*,1, Jean Logan2, Nora D Volkow3,4, Elena Shumay4, Fred McCall-Perez5, Millard Jayne4, Gene-Jack Wang4, David L Alexoff1, Karen Apelskog-Torres4, Barbara Hubbard1, Pauline Carter1, 1 6 7 4 1 1 4 Payton King , Stanley Fahn , Michelle Gilmor , Frank Telang , Colleen Shea , Youwen Xu and Lisa Muench 1Biological, Environmental and Climate Sciences Department, Brookhaven National Laboratory, Upton, NY, USA; 2New York University Langone Medical Center, Department of Radiology, New York, NY, USA; 3National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD, 4 5 USA; National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD, USA; Targeted Medical Pharma Inc, 6 7 Los Angeles, CA, USA; Department of Neurology, Columbia University Medical Center, New York, NY, USA; Novartis Pharmaceuticals, East Hanover, NJ, USA Selegiline (L-deprenyl) is a selective, irreversible inhibitor of monoamine oxidase B (MAO-B) at the conventional dose (10 mg/day oral) that is used in the treatment of Parkinson’s disease. However, controlled studies have demonstrated antidepressant activity for high doses of oral selegiline and for transdermal selegiline suggesting that when plasma levels of selegiline are elevated, brain MAO-A might also be inhibited. Zydis selegiline (Zelapar) is an orally disintegrating formulation of selegiline, which is absorbed through the buccal mucosa producing higher plasma levels of selegiline and reduced amphetamine metabolites compared with equal doses of conventional selegiline. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Antihistamine Therapy in Allergic Rhinitis
CLINICAL REVIEW Antihistamine Therapy in Allergic Rhinitis Paul R. Tarnasky, MD, and Paul P. Van Arsdel, Jr, MD Seattle, Washington Allergic rhinitis is a common disorder that is associated with a high incidence of mor bidity and considerable costs. The symptoms of allergic rhinitis are primarily depen dent upon the tissue effects of histamine. Antihistamines are the mainstay of therapy for allergic rhinitis. Recently, a second generation of antihistamines has become available. These agents lack the adverse effect of sedation, which is commonly associated with older antihistamines. Current practice of antihistamine therapy in allergic rhinitis often involves random selection among the various agents. Based upon the available clinical trials, chlorpheniramine appears to be the most reasonable initial antihistaminic agent. A nonsedating antihis tamine should be used initially if a patient is involved in activities where drowsiness is dangerous. In this comprehensive review of allergic rhinitis and its treatment, the cur rent as well as future options in antihistamine pharmacotherapy are emphasized. J Fam Pract 1990; 30:71-80. llergic rhinitis is a common condition afflicting some defined by the period of exposure to those agents to which A where between 15 and 30 million people in the United a patient is sensitive. Allergens in seasonal allergic rhinitis States.1-3 The prevalence of disease among adolescents is consist of pollens from nonflowering plants such as trees, estimated to be 20% to 30%. Two thirds of the adult grasses, and weeds. These pollens generally create symp allergic rhinitis patients are under 30 years of age.4-6 Con toms in early spring, late spring through early summer, sequently, considerable costs are incurred in days lost and fall, respectively. -

Potent Inhibition of Monoamine Oxidase B by a Piloquinone from Marine-Derived Streptomyces Sp. CNQ-027
J. Microbiol. Biotechnol. (2017), 27(4), 785–790 https://doi.org/10.4014/jmb.1612.12025 Research Article Review jmb Potent Inhibition of Monoamine Oxidase B by a Piloquinone from Marine-Derived Streptomyces sp. CNQ-027 Hyun Woo Lee1, Hansol Choi2, Sang-Jip Nam2, William Fenical3, and Hoon Kim1* 1Department of Pharmacy and Research Institute of Life Pharmaceutical Sciences, Sunchon National University, Suncheon 57922, Republic of Korea 2Department of Chemistry and Nano Science, Ewha Womans University, Seoul 03760, Republic of Korea 3Center for Marine Biotechnology and Biomedicine, Scripps Institution of Oceanography, University of California, San Diego, La Jolla, CA 92093-0204, USA Received: December 19, 2016 Revised: December 27, 2016 Two piloquinone derivatives isolated from Streptomyces sp. CNQ-027 were tested for the Accepted: January 4, 2017 inhibitory activities of two isoforms of monoamine oxidase (MAO), which catalyzes monoamine neurotransmitters. The piloquinone 4,7-dihydroxy-3-methyl-2-(4-methyl-1- oxopentyl)-6H-dibenzo[b,d]pyran-6-one (1) was found to be a highly potent inhibitor of First published online human MAO-B, with an IC50 value of 1.21 µM; in addition, it was found to be highly effective January 9, 2017 against MAO-A, with an IC50 value of 6.47 µM. Compound 1 was selective, but not extremely *Corresponding author so, for MAO-B compared with MAO-A, with a selectivity index value of 5.35. Compound 1,8- Phone: +82-61-750-3751; dihydroxy-2-methyl-3-(4-methyl-1-oxopentyl)-9,10-phenanthrenedione (2) was moderately Fax: +82-61-750-3708; effective for the inhibition of MAO-B (IC = 14.50 µM) but not for MAO-A (IC > 80 µM). -

Pre - PA Allowance Age 18 Years of Age Or Older Quantity 60 Grams Every 90 Days ______
DOXEPIN CREAM 5% (Prudoxin, Zonalon) Pre - PA Allowance Age 18 years of age or older Quantity 60 grams every 90 days _______________________________________________________________ Prior-Approval Requirements Age 18 years of age or older Diagnosis Patient must have the following: Moderate pruritus, due to atopic dermatitis (eczema) or lichen simplex chronicus AND the following: 1. Inadequate response, intolerance or contraindication to ONE medication in EACH of the following categories: a. Topical antihistamine (see Appendix I) b. High potency topical corticosteroid (see Appendix II) 2. Physician agrees to taper patient’s dose to the FDA recommended dose, and after tapered will only use for short-term pruritus relief (up to 8 days) a. Patients using over 60 grams of topical doxepin in 90 days be required to taper to 60 grams topical doxepin within 90 days Prior - Approval Limits Quantity 180 grams for 90 days Duration 3 months ___________________________________________________________________ Prior – Approval Renewal Requirements None (see appendix below) Doxepin 5% cream FEP Clinical Rationale DOXEPIN CREAM 5% (Prudoxin, Zonalon) APPENDIX I Drug Dosage Form Diphenhydramine Cream Phenyltoloxamine Lotion/ Cream Tripelennamine Cream Phendiamine Cream APPENDIX II Relative Potency of Selected Topical Corticosteroid Drug ProductsDosage Form Strength I. Very high potency Augmented betamethasone Ointment, Gel 0.05% dipropionate Clobetasol propionate Cream, Ointment 0.05% Diflorasone diacetate Ointment 0.05% Halobetasol propionate Cream, Ointment 0.05% II. High potency Amcinonide Cream, Lotion, 0.1% Augmented betamethasone Cream,Ointment Lotion 0.05% dipropionate Betamethasone Cream, Ointment 0.05% Betamethasonedipropionate valerate Ointment 0.1% Desoximetasone Cream, Ointment 0.25% Gel 0.05% Diflorasone diacetate Cream, Ointment 0.05% (emollient base) Fluocinonide Cream, Ointment, Gel 0.05% Halcinonide Cream, Ointment 0.1% Triamcinolone acetonide Cream, Ointment 0.5% III. -
![With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S](https://docslib.b-cdn.net/cover/2862/with-3h-mepyramine-trieyclic-antidepressants-antihistamine-neurotransmitter-amitriptyline-vinh-tan-tran-raymond-s-1512862.webp)
With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S
Proc. Nati. Acad. Sci. USA Vol. 75, No. 12, pp. 6290-6294,, December 1978 Neurobiology Histamine H1 receptors identified in mammalian brain membranes with [3H]mepyramine (trieyclic antidepressants/antihistamine/neurotransmitter/amitriptyline) VINH TAN TRAN, RAYMOND S. L. CHANG, AND SOLOMON H. SNYDER* Departments of Pharmacology and Experimental Therapeutics, and Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205 Communicated by Julius Axelrod, August 30,1978 ABSTRACT The antihistamine [3H mepyramine binds to Male Sprague-Dawley rats (150-200 g) were killed by cer- HI histamine receptors in mammalian brain membranes. vical dislocation, their brains were rapidly removed and ho- Potencies of H1 antihistamines at the binding sites correlate mogenized with a Polytron for 30 min (setting 5) in 30 vol of with their pharmacological antihistamine effects in the guinea pig ileum. Specific [3Himepyramine binding is saturable with ice-cold Na/K phosphate buffer (50 mM, pH 7.5), and the a dissociation constant of about 4 nM in both equilibrium and suspension was centrifuged (50,000 X g for 10 min). The pellet kinetic experiments and a density of 10pmolper gram ofwhole was resuspended in the same volume of fresh buffer and cen- brain. Some tricyclic antidepressants are potent inhibitors of trifuged, and the final pellet was resuspended in the original secific [3Hmepamine binding. Regional variations of volume of ice-cold buffer by Polytron homogenization. Calf [3Hjmepyramine ing do not correlate with variations in brains were obtained from a local abattoir within 2 hr after the endogeneous histamine and histidine decarboxylase activity. death of the animals and transferred to the laboratory in ice- Histamine is a neurotransmitter candidate in mammalian brain cold saline. -

The Effects of Albuterol and Chronic Antidepressants on Intracranial Self-Stimulation Reward Thresholds
University of Rhode Island DigitalCommons@URI Open Access Dissertations 1989 THE EFFECTS OF ALBUTEROL AND CHRONIC ANTIDEPRESSANTS ON INTRACRANIAL SELF-STIMULATION REWARD THRESHOLDS Robert Louis Dufresne University of Rhode Island Follow this and additional works at: https://digitalcommons.uri.edu/oa_diss Recommended Citation Dufresne, Robert Louis, "THE EFFECTS OF ALBUTEROL AND CHRONIC ANTIDEPRESSANTS ON INTRACRANIAL SELF-STIMULATION REWARD THRESHOLDS" (1989). Open Access Dissertations. Paper 146. https://digitalcommons.uri.edu/oa_diss/146 This Dissertation is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Dissertations by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. THE EFFECTS OF ALBUTEROL AND CHRONIC ANTIDEPRESSANTS ON INTRACRANIAL SELF-STIMULATION REWARD THRESHOLDS BY ROBERT LOUIS DUFRESNE A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF OOCTOR OF PHILOSOPHY IN PHARMACOLOGY AND TOXICOLOGY UNIVERSITY OF RHODE ISLAND 1989 DOCTOR OF PIDLOSOPHY DISSERTATION CF ROBERTLOUISDUFRESNE Approved: Dissertation Committee UNIVERSITY OF RHODE ISLAND 1989 Abstract Male Sprague Dawley rats implanted with bipolar stainless steel electrodes aimed at the medial forebrain bundle at the level of the hypothalamus were trained to self administer a rewarding current on a 20:10 DRP operant schedule. A procedure based on the psychophysical method of limits was used to determine the threshold of self administration for this stimulus. The effects of the B2-agonist albuterol (salbutamol) on threshold, rearing, and motor activity were examined prior to and after a 19 day period of daily administration of either desipramine 10 mg/kg, fluoxetine 10 mg/kg, or saline. -
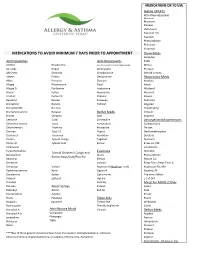
Medications to Avoid Minimum 7 Days Prior to Appointment
MEDICATIONS OK TO USE NASAL SPRAYS Afrin (Oxymetazoline) Atrovent Beconase Flonase Fluticasone Nasocort AQ Nasonex Phenylephrine Rhinocort Veramyst MEDICATIONS TO AVOID MINIMUM 7 DAYS PRIOR TO APPOINTMENT Derm Meds Atopiclair Antihistamines Anti-Depressants Elidel Actifed Novahistine (only at the advice of the prescribing physician) Mimyx Aerokids Nyquil Amitripyline Protopic AH-Chew Omnihist Clomipramine Steroid Creams Alavert Pediox Desipramine Respiratory Meds Aldex Periactin Doxepin Accolate Allegra Pheniramine Elavil Advair Allegra D Pyrilamine Imipramine Albuterol Atarax Pyrlex Norpramin Atrovent Atrohist Pyrlex CB Pamelor Flovent Benadryl Rescon Sinequan Pulmicort Bromphed Rondec Tofranil Singulair Bromphed PD Ru-Tuss Theophylline Brompheniramine Rynatan Reflux Meds Ventolin Brovex Semprex Axid Xopenex Cetirizine Sudal Cimetadine Decongestants/Expectorants Chlorpheniramine Tavist Famotadine Carbapentane Chlortrimeton Triaminic Nizatadine Delsym Clarinex Tussi-12 Pepcid Dextromethorphan Clarinex D Tussionex Ranitidine Duratuss Claritin Tylenol Allergy Tagamet Duravent Claritin D Tylenol Cold Zantac Entex LA, PSE Clemastine Guaifenisen Cyrproheptadine Humabid Tylenol Children's Cough and Eyedrops Deconamine Alocril Phenylephrine Runny Nose/Cold/Plus Flu Dexodryl Elestat Rescon GG Dimetane Livostin Respi-Tann, Respi-Tann G Dimetapp Vistaril Naphcon-A (Naphcon is ok) Robitussin PD, DM Diphenhydramine Xyzal Opcon-A Sudafed, PE Doxylamine Zyrtec Optichrome Triaminic Yellow Drixoral Zyrtec D Optivar Z-Cof DM Duradryl Pataday Meds for