The Influence of Histamine H1-Receptor on Liver Functions in Immunized Rabbits
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Human Histamine H2 Receptor, Frozen Cells Product No.: ES-391-AF
TECHNICAL AequoZen® DATA SHEET Research use only. Not for use in diagnostic procedures. You are authorized to utilize these frozen cell preparations one time only. Any attempt to transfer, re-use, or propagate these cells is expressly unauthorized and a violation of the product terms and conditions of sale. Human Histamine H2 Receptor, Frozen Cells Product No.: ES-391-AF Lot No.: 2562845 Material Provided Cells: 1 x 1 mL frozen aliquot Format: ~10 x 106 cells/mL in Ham’s F12, 10% FBS with 10 % DMSO Product Information Cellular Background: CHO-K1 Parental Frozen Cells (control): A19 (replaced with Cat # ES-000-A2F) Frozen Cells Info: Frozen recombinant, CHO-K1 cells expressing mitochondrially- targeted Aequorin, Gα16 and the human Histamine H2 receptor. DNA Sequence: Identical to coding sequence of GenBank NM_022304.2. Corresponding Protein Sequence: Identical to GenBank NP_071640.1. Storage Conditions: Store in liquid nitrogen (vapor phase) immediately upon receipt, or maximum 15 days at -80°C. AequoZen® is designed for single use only. Do not refreeze. Quality Control ® EC50 for a reference agonist is determined using an AequoScreen assay (Figure 1). Mycoplasma test is performed using MycoAlert® Mycoplasma detection kit. We certify that these results meet our quality release criteria. Amthamine dihydrobromide (EC50): 6.9 nM Mycoplasma: This cell line tested negative for Mycoplasma. TDS-ES-391-AF-04 Page 1 of 5 Recommended Thawing Conditions and Handling of Frozen Cells Carefully follow instructions below to obtain the expected results. Most Frozen cells are intended to be assayed immediately upon thawing. Exceptionally, where specified, some frozen cell products require an overnight incubation in Cell Medium to enable them to perform optimally. -

Opioid and Other Substance Use Disorders
OPIOID AND OTHER SUBSTANCE USE DISODERS Dr Amit Arya Additional Professor Department of Psychiatry KGMU Lucknow What are addictive substances? Any substance which when taken has an ability to change the person’s consciousness, thinking, mood, behaviour and motor functions Leading to take the substance repeatedly (World Health Organisation, 1992) …Also called as psychoactive substances Layman term: “Drugs” Why are certain substances addictive? Intake of any substance – oral, inhalational, injecting Enters the bloodstream Acts on a specific body part, such as heart, lung, stomach, etc. Addictive substances act on brain Addictive Substances act on brain All substances acting on the brain are not addictive Addictive substances I want to primarily act on a particular take that area/group of neurons in the drug again! brain, Leading the individual to repeatedly administer the addictive / psychoactive substance → “drug seeking” behaviour Addictive substances primarily act on a particular area/group of neurons in the brain. Regions controlling emotions, thinking, Frontal region judgement & memory Mid Brain How are addictive substances different from each other? Broad actions Chemical that the drug class of drugs produces on the brain Source of drug Natural/semi- TYPOLOGY synthetic/synthetic Mode of intake Oral/inhalational/ Availability – parenteral legal/illegal? (injections) Typology – Chemical Class Opioids Alcohol Cannabis Volatile solvents Based on chemical class Stimulants Tobacco Sedative- hypnotics Hallucinogens The usual drug-use -

Chapter 2 Molecular Aspects of Histamine Receptors
VU Research Portal Shedding Light on the Histamine H3 Receptor Mocking, T.A.M. 2020 document version Publisher's PDF, also known as Version of record Link to publication in VU Research Portal citation for published version (APA) Mocking, T. A. M. (2020). Shedding Light on the Histamine H3 Receptor: Photopharmacology and bioluminescent assays to study GPCRs. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. E-mail address: [email protected] Download date: 06. Oct. 2021 Chapter 2 Molecular aspects of histamine receptors Histamine mediates a multitude of physiological effects in the human body by activating four histamine receptor subtypes. Histamine receptors have proven to be promising drug targets in the treatment of a variety of diseases, including hay fever, gastric ulcers, inflammatory and neuropathological diseases. In this chapter the molecular aspects of histamine receptors are described, including expression profile, intracellular signaling, and how histamine receptor activity can be attenuated by ligands targeting the histamine receptor binding sites. -

Histamine Receptors
Tocris Scientific Review Series Tocri-lu-2945 Histamine Receptors Iwan de Esch and Rob Leurs Introduction Leiden/Amsterdam Center for Drug Research (LACDR), Division Histamine is one of the aminergic neurotransmitters and plays of Medicinal Chemistry, Faculty of Sciences, Vrije Universiteit an important role in the regulation of several (patho)physiological Amsterdam, De Boelelaan 1083, 1081 HV, Amsterdam, The processes. In the mammalian brain histamine is synthesised in Netherlands restricted populations of neurons that are located in the tuberomammillary nucleus of the posterior hypothalamus.1 Dr. Iwan de Esch is an assistant professor and Prof. Rob Leurs is These neurons project diffusely to most cerebral areas and have full professor and head of the Division of Medicinal Chemistry of been implicated in several brain functions (e.g. sleep/ the Leiden/Amsterdam Center of Drug Research (LACDR), VU wakefulness, hormonal secretion, cardiovascular control, University Amsterdam, The Netherlands. Since the seventies, thermoregulation, food intake, and memory formation).2 In histamine receptor research has been one of the traditional peripheral tissues, histamine is stored in mast cells, eosinophils, themes of the division. Molecular understanding of ligand- basophils, enterochromaffin cells and probably also in some receptor interaction is obtained by combining pharmacology specific neurons. Mast cell histamine plays an important role in (signal transduction, proliferation), molecular biology, receptor the pathogenesis of various allergic conditions. After mast cell modelling and the synthesis and identification of new ligands. degranulation, release of histamine leads to various well-known symptoms of allergic conditions in the skin and the airway system. In 1937, Bovet and Staub discovered compounds that antagonise the effect of histamine on these allergic reactions.3 Ever since, there has been intense research devoted towards finding novel ligands with (anti-) histaminergic activity. -

Physiological Implications of Biased Signaling at Histamine H2 Receptors
ORIGINAL RESEARCH published: 10 March 2015 doi: 10.3389/fphar.2015.00045 Physiological implications of biased signaling at histamine H2 receptors Natalia Alonso 1,2,CarlosD.Zappia2,3, Maia Cabrera 2,3, Carlos A. Davio 2,3,4 , Carina Shayo 1,2, Federico Monczor 2,3 and Natalia C. Fernández 2,3* 1 Laboratorio de Patología y Farmacología Molecular, Instituto de Biología y Medicina Experimental, Buenos Aires, Argentina, 2 Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina, 3 Laboratorio de Farmacología de Receptores, Cátedra de Química Medicinal, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Buenos Aires, Argentina, 4 Instituto de Investigaciones Farmacológicas – Universidad de Buenos Aires – Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina Histamine mediates numerous functions acting through its four receptor subtypes all belonging to the large family of seven transmembrane G-protein coupled receptors. In particular, histamine H2 receptor (H2R) is mainly involved in gastric acid production, becoming a classic pharmacological target to treat Zollinger–Ellison disease and gastric Edited by: Claudio M. Costa-Neto, and duodenal ulcers. H2 ligands rank among the most widely prescribed and over University of São Paulo, Brazil the counter-sold drugs in the world. Recent evidence indicate that some H2R ligands Reviewed by: display biased agonism, selecting and triggering some, but not all, of the signaling Terry Kenakin, pathways associated to the H2R. The aim of the present work is to study whether University of North Carolina Chapel Hill, USA famotidine, clinically widespread used ligand acting at H2R, exerts biased signaling. Our Andre Sampaio Pupo, findings indicate that while famotidine acts as inverse agonist diminishing cAMP basal São Paulo State University, Brazil levels, it mimics the effects of histamine and the agonist amthamine concerning receptor *Correspondence: Natalia C. -

(12) United States Patent (10) Patent No.: US 8,486,621 B2 Luo Et Al
USOO8486.621B2 (12) United States Patent (10) Patent No.: US 8,486,621 B2 Luo et al. (45) Date of Patent: Jul. 16, 2013 (54) NUCLEICACID-BASED MATRIXES 2005. O130180 A1 6/2005 Luo et al. 2006/0084607 A1 4/2006 Spirio et al. (75) Inventors: ity, N. (US); Soong Ho 2007/01482462007/0048759 A1 3/20076/2007 Luo et al. m, Ithaca, NY (US) 2008.0167454 A1 7, 2008 Luo et al. 2010, O136614 A1 6, 2010 Luo et al. (73) Assignee: Cornell Research Foundation, Inc., 2012/0022244 A1 1, 2012 Yin Ithaca, NY (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this WO WO 2004/057023 A1 T 2004 patent is extended or adjusted under 35 U.S.C. 154(b) by 808 days. OTHER PUBLICATIONS Lin et al. (J Biomech Eng. Feb. 2004;126(1):104-10).* (21) Appl. No.: 11/464,184 Li et al. (Nat Mater. Jan. 2004:3(1):38-42. Epub Dec. 21, 2003).* Ma et al. (Nucleic Acids Res. Dec. 22, 1986;14(24):9745-53).* (22) Filed: Aug. 11, 2006 Matsuura, et al. Nucleo-nanocages: designed ternary oligodeoxyribonucleotides spontaneously form nanosized DNA (65) Prior Publication Data cages. Chem Commun (Camb). 2003; (3):376-7. Li, et al. Multiplexed detection of pathogen DNA with DNA-based US 2007/01 17177 A1 May 24, 2007 fluorescence nanobarcodes. Nat Biotechnol. 2005; 23(7): 885-9. Lund, et al. Self-assembling a molecular pegboard. JAm ChemSoc. Related U.S. Application Data 2005; 127(50): 17606-7. (60) Provisional application No. 60/722,032, filed on Sep. -

Antihistamine Therapy in Allergic Rhinitis
CLINICAL REVIEW Antihistamine Therapy in Allergic Rhinitis Paul R. Tarnasky, MD, and Paul P. Van Arsdel, Jr, MD Seattle, Washington Allergic rhinitis is a common disorder that is associated with a high incidence of mor bidity and considerable costs. The symptoms of allergic rhinitis are primarily depen dent upon the tissue effects of histamine. Antihistamines are the mainstay of therapy for allergic rhinitis. Recently, a second generation of antihistamines has become available. These agents lack the adverse effect of sedation, which is commonly associated with older antihistamines. Current practice of antihistamine therapy in allergic rhinitis often involves random selection among the various agents. Based upon the available clinical trials, chlorpheniramine appears to be the most reasonable initial antihistaminic agent. A nonsedating antihis tamine should be used initially if a patient is involved in activities where drowsiness is dangerous. In this comprehensive review of allergic rhinitis and its treatment, the cur rent as well as future options in antihistamine pharmacotherapy are emphasized. J Fam Pract 1990; 30:71-80. llergic rhinitis is a common condition afflicting some defined by the period of exposure to those agents to which A where between 15 and 30 million people in the United a patient is sensitive. Allergens in seasonal allergic rhinitis States.1-3 The prevalence of disease among adolescents is consist of pollens from nonflowering plants such as trees, estimated to be 20% to 30%. Two thirds of the adult grasses, and weeds. These pollens generally create symp allergic rhinitis patients are under 30 years of age.4-6 Con toms in early spring, late spring through early summer, sequently, considerable costs are incurred in days lost and fall, respectively. -

International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors
1521-0081/67/3/601–655$25.00 http://dx.doi.org/10.1124/pr.114.010249 PHARMACOLOGICAL REVIEWS Pharmacol Rev 67:601–655, July 2015 Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics ASSOCIATE EDITOR: ELIOT H. OHLSTEIN International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors Pertti Panula, Paul L. Chazot, Marlon Cowart, Ralf Gutzmer, Rob Leurs, Wai L. S. Liu, Holger Stark, Robin L. Thurmond, and Helmut L. Haas Department of Anatomy, and Neuroscience Center, University of Helsinki, Finland (P.P.); School of Biological and Biomedical Sciences, University of Durham, United Kingdom (P.L.C.); AbbVie, Inc. North Chicago, Illinois (M.C.); Department of Dermatology and Allergy, Hannover Medical School, Hannover, Germany (R.G.); Department of Medicinal Chemistry, Amsterdam Institute of Molecules, Medicines and Systems, VU University Amsterdam, The Netherlands (R.L.); Ziarco Pharma Limited, Canterbury, United Kingdom (W.L.S.L.); Institute of Pharmaceutical and Medical Chemistry (H.S.) and Institute of Neurophysiology, Medical Faculty (H.L.H.), Heinrich-Heine-University Duesseldorf, Germany; and Janssen Research & Development, LLC, San Diego, California (R.L.T.) Abstract ....................................................................................602 Downloaded from I. Introduction and Historical Perspective .....................................................602 II. Histamine H1 Receptor . ..................................................................604 A. Receptor Structure -
![With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S](https://docslib.b-cdn.net/cover/2862/with-3h-mepyramine-trieyclic-antidepressants-antihistamine-neurotransmitter-amitriptyline-vinh-tan-tran-raymond-s-1512862.webp)
With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S
Proc. Nati. Acad. Sci. USA Vol. 75, No. 12, pp. 6290-6294,, December 1978 Neurobiology Histamine H1 receptors identified in mammalian brain membranes with [3H]mepyramine (trieyclic antidepressants/antihistamine/neurotransmitter/amitriptyline) VINH TAN TRAN, RAYMOND S. L. CHANG, AND SOLOMON H. SNYDER* Departments of Pharmacology and Experimental Therapeutics, and Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205 Communicated by Julius Axelrod, August 30,1978 ABSTRACT The antihistamine [3H mepyramine binds to Male Sprague-Dawley rats (150-200 g) were killed by cer- HI histamine receptors in mammalian brain membranes. vical dislocation, their brains were rapidly removed and ho- Potencies of H1 antihistamines at the binding sites correlate mogenized with a Polytron for 30 min (setting 5) in 30 vol of with their pharmacological antihistamine effects in the guinea pig ileum. Specific [3Himepyramine binding is saturable with ice-cold Na/K phosphate buffer (50 mM, pH 7.5), and the a dissociation constant of about 4 nM in both equilibrium and suspension was centrifuged (50,000 X g for 10 min). The pellet kinetic experiments and a density of 10pmolper gram ofwhole was resuspended in the same volume of fresh buffer and cen- brain. Some tricyclic antidepressants are potent inhibitors of trifuged, and the final pellet was resuspended in the original secific [3Hmepamine binding. Regional variations of volume of ice-cold buffer by Polytron homogenization. Calf [3Hjmepyramine ing do not correlate with variations in brains were obtained from a local abattoir within 2 hr after the endogeneous histamine and histidine decarboxylase activity. death of the animals and transferred to the laboratory in ice- Histamine is a neurotransmitter candidate in mammalian brain cold saline. -

WO 2011/089216 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date t 28 July 2011 (28.07.2011) WO 2011/089216 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 47/48 (2006.01) C07K 1/13 (2006.01) kind of national protection available): AE, AG, AL, AM, C07K 1/1 07 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) Number: International Application DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP201 1/050821 HN, HR, HU, ID, J , IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 2 1 January 201 1 (21 .01 .201 1) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 1015 1465. 1 22 January 2010 (22.01 .2010) EP kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, (71) Applicant (for all designated States except US): AS- ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, CENDIS PHARMA AS [DK/DK]; Tuborg Boulevard TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, 12, DK-2900 Hellerup (DK). -

Some Pharmacological Properties of Trimethoxy Analogs of Pheniramine, Tripelennamine, and Diphenhydramine
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 1966 Some Pharmacological Properties of Trimethoxy Analogs of Pheniramine, Tripelennamine, and Diphenhydramine Ronald Otto Langner University of Rhode Island Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Langner, Ronald Otto, "Some Pharmacological Properties of Trimethoxy Analogs of Pheniramine, Tripelennamine, and Diphenhydramine" (1966). Open Access Master's Theses. Paper 200. https://digitalcommons.uri.edu/theses/200 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. SOME PHARMACOLOGICAL PRDPERTIES OF TRIMETHOXY ANALOGS OF PHENIRAMINE,_ TRIPELENNAMINE, AND DIPHENHYDRAMINE BY RONALD OT'ID LANGNER A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FDR THE DEGREE OF MASTER OF SCIENCE IN PHARMACOLOGY UNIVERSITY OF RHODE ISLAND 1966 ABSTRACT Trimethoxy analogs of tripelennamine, diphenhydramine, and pheniramine were studied to determine the influences of the tri methoxy group on the pharmacological activity of known antihista mines. In this investigation, the ability of the parent compounds and their analogs to antagonize the histamine-induced contractions of guinea pig ilia were studied as well as their effects on the systolic blood pressure of male albino rats and on the central nervous system of mice as measured by the actophotometer. 'lhe trimethoxy analogs were comparatively weak competitive antagonists of histamine with the exception of N,N,-Diethyl-N' (2-pyridyl)-N'-(3,4,S-trimethoxybenzyl) ethylenediamine Dicyclamate (De-TMPBZ) and N,N,-Diethyl-N'-(2-pyridyl)-N'-(3,4,5-trimethoxy benzyl) ethylenediamine Disuccinate (TMPBZ) which were weak non competitive histamine antagonists. -
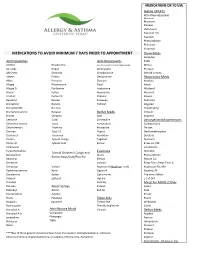
Medications to Avoid Minimum 7 Days Prior to Appointment
MEDICATIONS OK TO USE NASAL SPRAYS Afrin (Oxymetazoline) Atrovent Beconase Flonase Fluticasone Nasocort AQ Nasonex Phenylephrine Rhinocort Veramyst MEDICATIONS TO AVOID MINIMUM 7 DAYS PRIOR TO APPOINTMENT Derm Meds Atopiclair Antihistamines Anti-Depressants Elidel Actifed Novahistine (only at the advice of the prescribing physician) Mimyx Aerokids Nyquil Amitripyline Protopic AH-Chew Omnihist Clomipramine Steroid Creams Alavert Pediox Desipramine Respiratory Meds Aldex Periactin Doxepin Accolate Allegra Pheniramine Elavil Advair Allegra D Pyrilamine Imipramine Albuterol Atarax Pyrlex Norpramin Atrovent Atrohist Pyrlex CB Pamelor Flovent Benadryl Rescon Sinequan Pulmicort Bromphed Rondec Tofranil Singulair Bromphed PD Ru-Tuss Theophylline Brompheniramine Rynatan Reflux Meds Ventolin Brovex Semprex Axid Xopenex Cetirizine Sudal Cimetadine Decongestants/Expectorants Chlorpheniramine Tavist Famotadine Carbapentane Chlortrimeton Triaminic Nizatadine Delsym Clarinex Tussi-12 Pepcid Dextromethorphan Clarinex D Tussionex Ranitidine Duratuss Claritin Tylenol Allergy Tagamet Duravent Claritin D Tylenol Cold Zantac Entex LA, PSE Clemastine Guaifenisen Cyrproheptadine Humabid Tylenol Children's Cough and Eyedrops Deconamine Alocril Phenylephrine Runny Nose/Cold/Plus Flu Dexodryl Elestat Rescon GG Dimetane Livostin Respi-Tann, Respi-Tann G Dimetapp Vistaril Naphcon-A (Naphcon is ok) Robitussin PD, DM Diphenhydramine Xyzal Opcon-A Sudafed, PE Doxylamine Zyrtec Optichrome Triaminic Yellow Drixoral Zyrtec D Optivar Z-Cof DM Duradryl Pataday Meds for