HPLC Determination and Quantification of Pheniramine And
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Opioid and Other Substance Use Disorders
OPIOID AND OTHER SUBSTANCE USE DISODERS Dr Amit Arya Additional Professor Department of Psychiatry KGMU Lucknow What are addictive substances? Any substance which when taken has an ability to change the person’s consciousness, thinking, mood, behaviour and motor functions Leading to take the substance repeatedly (World Health Organisation, 1992) …Also called as psychoactive substances Layman term: “Drugs” Why are certain substances addictive? Intake of any substance – oral, inhalational, injecting Enters the bloodstream Acts on a specific body part, such as heart, lung, stomach, etc. Addictive substances act on brain Addictive Substances act on brain All substances acting on the brain are not addictive Addictive substances I want to primarily act on a particular take that area/group of neurons in the drug again! brain, Leading the individual to repeatedly administer the addictive / psychoactive substance → “drug seeking” behaviour Addictive substances primarily act on a particular area/group of neurons in the brain. Regions controlling emotions, thinking, Frontal region judgement & memory Mid Brain How are addictive substances different from each other? Broad actions Chemical that the drug class of drugs produces on the brain Source of drug Natural/semi- TYPOLOGY synthetic/synthetic Mode of intake Oral/inhalational/ Availability – parenteral legal/illegal? (injections) Typology – Chemical Class Opioids Alcohol Cannabis Volatile solvents Based on chemical class Stimulants Tobacco Sedative- hypnotics Hallucinogens The usual drug-use -

Diphenhydramine Hydrochloride (CASRN 147-24-0) in F344/N Rats
NATIONAL TOXICOLOGY PROGRAM Technical Report Series No. 355 TOXICOLOGY AND CARCINOGENESIS STUDIES OF DIPHENHYDRAMINE HYDROCHLORIDE (CAS NO. 147-24-0) IN F344/N RATS AND B6C3F1 MICE (FEED STUDIES) LJ.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health NTP ‘TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF DIPHENHYDRAMINE HYDROCHLORIDE (CAS NO. 147-24-0) IN F344/N RATS AND B6C3F1 MICE (FEED STUDIES) R. Melnick, Ph.D., Study Scientist NATIONAL TOXICOLOGY PROGRAM P.O. Box 12233 Research Triangle Park, NC 27709 September 1989 NTP TR 355 NIH Publication No. 89-2810 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health CONTENTS PAGE ABSTRACT ................................................................ 3 EXPLANATION OF LEVELS OF EVIDENCE OF CARCINOGENIC ACTIVITY .................. 6 CONTRIBUTORS ............................................................ 7 PEERREVIEWPANEL ........................................................ 8 SUMMARY OF PEER REVIEW COMMENTS ......................................... 9 I. INTRODUCTION ........................................................ 11 I1. MATERIALS AND METHODS .............................................. 21 III. RESULTS ............................................................. 35 RATS ............................................................. 36 MICE ............................................................. 45 GENETIC TOXICOLOGY ............................................... 53 IV. -

Methapyrilene Hydrochloride (CAS No
National Toxicology Program Toxicity Report Series Number 46 NTP Technical Report on the Hepatotoxicity Studies of the Liver Carcinogen Methapyrilene Hydrochloride (CAS No. 135-23-9) Administered in Feed to Male F344/N Rats July 2000 U.S. Department of Health and Human Services Public Health Service National Institutes of Health FOREWORD The National Toxicology Program (NTP) is made up of four charter agencies of the U.S. Department of Health and Human Services (DHHS): the National Cancer Institute (NCI), National Institutes of Health; the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health; the National Center for Toxicological Research (NCTR), Food and Drug Administration; and the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. In July 1981, the Carcinogenesis Bioassay Testing Program, NCI, was transferred to the NIEHS. The NTP coordinates the relevant programs, staff, and resources from these Public Health Service agencies relating to basic and applied research and to biological assay development and validation. The NTP develops, evaluates, and disseminates scientific information about potentially toxic and hazardous chemicals. This knowledge is used for protecting the health of the American people and for the primary prevention of disease. The studies described in this Toxicity Study Report were performed under the direction of the NIEHS and were conducted in compliance with NTP laboratory health and safety requirements and must meet or exceed all applicable federal, state, and local health and safety regulations. Animal care and use were in accordance with the Public Health Service Policy on Humane Care and Use of Animals. -

Antihistamine Therapy in Allergic Rhinitis
CLINICAL REVIEW Antihistamine Therapy in Allergic Rhinitis Paul R. Tarnasky, MD, and Paul P. Van Arsdel, Jr, MD Seattle, Washington Allergic rhinitis is a common disorder that is associated with a high incidence of mor bidity and considerable costs. The symptoms of allergic rhinitis are primarily depen dent upon the tissue effects of histamine. Antihistamines are the mainstay of therapy for allergic rhinitis. Recently, a second generation of antihistamines has become available. These agents lack the adverse effect of sedation, which is commonly associated with older antihistamines. Current practice of antihistamine therapy in allergic rhinitis often involves random selection among the various agents. Based upon the available clinical trials, chlorpheniramine appears to be the most reasonable initial antihistaminic agent. A nonsedating antihis tamine should be used initially if a patient is involved in activities where drowsiness is dangerous. In this comprehensive review of allergic rhinitis and its treatment, the cur rent as well as future options in antihistamine pharmacotherapy are emphasized. J Fam Pract 1990; 30:71-80. llergic rhinitis is a common condition afflicting some defined by the period of exposure to those agents to which A where between 15 and 30 million people in the United a patient is sensitive. Allergens in seasonal allergic rhinitis States.1-3 The prevalence of disease among adolescents is consist of pollens from nonflowering plants such as trees, estimated to be 20% to 30%. Two thirds of the adult grasses, and weeds. These pollens generally create symp allergic rhinitis patients are under 30 years of age.4-6 Con toms in early spring, late spring through early summer, sequently, considerable costs are incurred in days lost and fall, respectively. -

A Comparison of Drug Use in Driver Fatalities and Similarly Exposed Drivers
DOT HS-802 488 A COMPARISON OF DRUG USE IN DRIVER FATALITIES AND SIMILARLY EXPOSED DRIVERS Contract No. DOT-HS-4-00941 July 1977 Final Report PREPARED FOR: U.S. DEPARTMENT OF TRANSPORTATION NATIONAL HIGHWAY TRAFFIC SAFETY ADMINISTRATION WASHINGTON, D.C. 20590 Document is available to the public through the National Technical Information Service, Springfield, Virginia 22161 This document is disseminated under the sponsorship of the Department of Transportation in the interest of information exchange. The United States Govern ment assumes no liability for its contents or use thereof. Technical Report Documentation Page 1. Report No. 2. Goeemm.nt Accession No. 3. Recipient's Catalog No. DOT HS-802 488 -^. 1. Title and Subtitle 5. Report Date July 1977 A COMPARISON OF DRU^ USE IN DRIVER FATALITIES 6. Performing Organization Code AND SIMILARLY EXPOSED DRIVERS . B. Performing Organization Report No. 7. Authorrs) Robert R. Blackburn, Edward J. Woodhouse 3963-D 9. Performing Organization Name and Address 10. Worb Unit No. (TRAIS) Midwest Research Institute I). Contract at Grant No. 425 Volker Boulevard DOT-HS-4-00941 Kansas City, Missouri 64110 13. Type of Report and Period Covered 12. Sponsoring Agency Name and Address 28 June 1974-25 March 1977 i U.S. Department of Transportation National Highway Traffic Safety Administration Final Report Washington, D.C. 20590 14. Sponsoring Agency Code 15. supplementary Notes 16. Abstract Crash information, urine, blood and bile samples from 900 fatally injured drivers were collected by medical examiners in 22 areas of the country. Ran domly selected living drivers were interviewed at times and places of recent fatal crashes in Dallas, Texas, and Memphis, Tennessee and breath, urine, and blood samples were obtained. -
![With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S](https://docslib.b-cdn.net/cover/2862/with-3h-mepyramine-trieyclic-antidepressants-antihistamine-neurotransmitter-amitriptyline-vinh-tan-tran-raymond-s-1512862.webp)
With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S
Proc. Nati. Acad. Sci. USA Vol. 75, No. 12, pp. 6290-6294,, December 1978 Neurobiology Histamine H1 receptors identified in mammalian brain membranes with [3H]mepyramine (trieyclic antidepressants/antihistamine/neurotransmitter/amitriptyline) VINH TAN TRAN, RAYMOND S. L. CHANG, AND SOLOMON H. SNYDER* Departments of Pharmacology and Experimental Therapeutics, and Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205 Communicated by Julius Axelrod, August 30,1978 ABSTRACT The antihistamine [3H mepyramine binds to Male Sprague-Dawley rats (150-200 g) were killed by cer- HI histamine receptors in mammalian brain membranes. vical dislocation, their brains were rapidly removed and ho- Potencies of H1 antihistamines at the binding sites correlate mogenized with a Polytron for 30 min (setting 5) in 30 vol of with their pharmacological antihistamine effects in the guinea pig ileum. Specific [3Himepyramine binding is saturable with ice-cold Na/K phosphate buffer (50 mM, pH 7.5), and the a dissociation constant of about 4 nM in both equilibrium and suspension was centrifuged (50,000 X g for 10 min). The pellet kinetic experiments and a density of 10pmolper gram ofwhole was resuspended in the same volume of fresh buffer and cen- brain. Some tricyclic antidepressants are potent inhibitors of trifuged, and the final pellet was resuspended in the original secific [3Hmepamine binding. Regional variations of volume of ice-cold buffer by Polytron homogenization. Calf [3Hjmepyramine ing do not correlate with variations in brains were obtained from a local abattoir within 2 hr after the endogeneous histamine and histidine decarboxylase activity. death of the animals and transferred to the laboratory in ice- Histamine is a neurotransmitter candidate in mammalian brain cold saline. -

Some Pharmacological Properties of Trimethoxy Analogs of Pheniramine, Tripelennamine, and Diphenhydramine
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 1966 Some Pharmacological Properties of Trimethoxy Analogs of Pheniramine, Tripelennamine, and Diphenhydramine Ronald Otto Langner University of Rhode Island Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Langner, Ronald Otto, "Some Pharmacological Properties of Trimethoxy Analogs of Pheniramine, Tripelennamine, and Diphenhydramine" (1966). Open Access Master's Theses. Paper 200. https://digitalcommons.uri.edu/theses/200 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. SOME PHARMACOLOGICAL PRDPERTIES OF TRIMETHOXY ANALOGS OF PHENIRAMINE,_ TRIPELENNAMINE, AND DIPHENHYDRAMINE BY RONALD OT'ID LANGNER A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FDR THE DEGREE OF MASTER OF SCIENCE IN PHARMACOLOGY UNIVERSITY OF RHODE ISLAND 1966 ABSTRACT Trimethoxy analogs of tripelennamine, diphenhydramine, and pheniramine were studied to determine the influences of the tri methoxy group on the pharmacological activity of known antihista mines. In this investigation, the ability of the parent compounds and their analogs to antagonize the histamine-induced contractions of guinea pig ilia were studied as well as their effects on the systolic blood pressure of male albino rats and on the central nervous system of mice as measured by the actophotometer. 'lhe trimethoxy analogs were comparatively weak competitive antagonists of histamine with the exception of N,N,-Diethyl-N' (2-pyridyl)-N'-(3,4,S-trimethoxybenzyl) ethylenediamine Dicyclamate (De-TMPBZ) and N,N,-Diethyl-N'-(2-pyridyl)-N'-(3,4,5-trimethoxy benzyl) ethylenediamine Disuccinate (TMPBZ) which were weak non competitive histamine antagonists. -
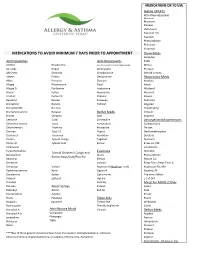
Medications to Avoid Minimum 7 Days Prior to Appointment
MEDICATIONS OK TO USE NASAL SPRAYS Afrin (Oxymetazoline) Atrovent Beconase Flonase Fluticasone Nasocort AQ Nasonex Phenylephrine Rhinocort Veramyst MEDICATIONS TO AVOID MINIMUM 7 DAYS PRIOR TO APPOINTMENT Derm Meds Atopiclair Antihistamines Anti-Depressants Elidel Actifed Novahistine (only at the advice of the prescribing physician) Mimyx Aerokids Nyquil Amitripyline Protopic AH-Chew Omnihist Clomipramine Steroid Creams Alavert Pediox Desipramine Respiratory Meds Aldex Periactin Doxepin Accolate Allegra Pheniramine Elavil Advair Allegra D Pyrilamine Imipramine Albuterol Atarax Pyrlex Norpramin Atrovent Atrohist Pyrlex CB Pamelor Flovent Benadryl Rescon Sinequan Pulmicort Bromphed Rondec Tofranil Singulair Bromphed PD Ru-Tuss Theophylline Brompheniramine Rynatan Reflux Meds Ventolin Brovex Semprex Axid Xopenex Cetirizine Sudal Cimetadine Decongestants/Expectorants Chlorpheniramine Tavist Famotadine Carbapentane Chlortrimeton Triaminic Nizatadine Delsym Clarinex Tussi-12 Pepcid Dextromethorphan Clarinex D Tussionex Ranitidine Duratuss Claritin Tylenol Allergy Tagamet Duravent Claritin D Tylenol Cold Zantac Entex LA, PSE Clemastine Guaifenisen Cyrproheptadine Humabid Tylenol Children's Cough and Eyedrops Deconamine Alocril Phenylephrine Runny Nose/Cold/Plus Flu Dexodryl Elestat Rescon GG Dimetane Livostin Respi-Tann, Respi-Tann G Dimetapp Vistaril Naphcon-A (Naphcon is ok) Robitussin PD, DM Diphenhydramine Xyzal Opcon-A Sudafed, PE Doxylamine Zyrtec Optichrome Triaminic Yellow Drixoral Zyrtec D Optivar Z-Cof DM Duradryl Pataday Meds for -

AVIL® Pheniramine Maleate Tablets & Injection
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory AVIL® Pheniramine Maleate Tablets & Injection THERAPEUTIC CATEGORY Antihistaminic COMPOSITION AVIL 25 Each uncoated tablet contains: Pheniramine Maleate IP…25mg AVIL 50 Each uncoated tablet contains: Pheniramine Maleate IP…50mg AVIL Injection (in 2ml ampoule) Each ml contains: Pheniramine Maleate IP …22.75mg AVIL Injection (in 10ml vial) Each ml contains: Pheniramine Maleate IP …… 22.75mg Methyl-Parahydroxybenzoate IP ……0.135%w/v Propyl-Parahydroxybenzoate IP ……0.015%w/v Water for Injection ………… q.s THERAPEUTIC INDICATIONS Allergic conditions (Hay fever, drug rashes, angioneurotic oedema, serum sickness, allergic conjunctivitis, food allergy etc.), Conditions of the respiratory tract that are accompanied by increased secretion, All itching skin conditions, Prevention and treatment of motion sickness, nausea, vomiting and vertigo due to Menière’s disease and other labyrinthine disturbances. DOSAGE AND ADMINISTRATION The dosage should be adjusted individually. When immediate or rapid response is desired, intravenous or intramuscular Avil injection should be used. Tablets: Adults & Children (above 10 years): Initially 1 tablet of Avil 25 two to three times a day. The dose may be increased to two tablets of Avil 25 or one tablet of Avil 50, administered two to three times a day if needed. The maximum dose of 3mg/kg/day should not be exceeded. Children 5-10 years of age: Half a tablet up to three times daily. Avil® tablets are not recommended in children under 5 years of age. Do not take the medicine on an empty stomach. For travel sickness, the dose should be taken atleast 30 mins before travelling. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Antihistamines on Zirchrom®-PS
Technical Bulletin #250 Separation of Antihistamines mAU Analytes 120 1 - Pheniramine 100 2 - Doxylamine 3 - Chlorpheniramine 80 4 - Methapyrilene 5 - Tripelennamine 60 6 - Cyclizine 40 2 3 4 1 5 20 6 0 -20 0 1 2 3 4 5 6 7 LC Conditions Column: -C18, 100 × 4.6 mm Mobile Phase: 10/25/65 A/B/C Flow rate: 1.0 mL/min. A: ACN Temperature: 75 ºC B: THF Injection volume: 5 µL C: 50mM Tetramethylammonium hydroxide, Detection: 254 nm 10mM Triethylamine, pH 12.2 Technical Bulletin #263 Antihistamines on ZirChrom®-PS mAU 4 175 Meclizine 150 Cyclizine 5 125 Cl N 100 1 N N 2 75 N 50 3 25 0 0 1 2 3 4 5 min Analytes 1 - Tripelennamine 2 - Triprolidine 3 - Cyclizine 4 - Pyrrobutamine 5 - Meclizine LC Conditions Column: ZirChrom®-PS, 50 mm × 4.6 mm i.d. Flow rate: 1.0 mL/min. Mobile Phase: Gradient Elution Temperature: 40 ºC Injection volume: 0.5 µL Time (Minutes) % A %B Detection: 254 nm 0 95 5 Back Pressure: 56 bar 1 85 15 4 40 60 A: 25mM HCl, pH 1.9 B: ACN Faster Analysis and Higher Efficiency with Thermally- Stable HPLC Columns Technical Bulletin #269 Dwight Stoll, Peter W. Carr ZirChrom Separations, Inc. Chromatographers have long known that modest increases in The initial separation is shown in Figure 1. Then, the temperature operating temperature can dramatically improve both the was increased to 50 oC, and the eluent flow rate also increased to efficiency and speed of an HPLC separation. Until now, this maintain the same system backpressure. -

Autacoids Introduction
AUTACOIDS INTRODUCTION • AUTACOIDS auto=self akos=healing/remedy • Local Hormones CLASSIFICATION • Amine derived: Histamine (amino acid: Histidine), Serotonin (Tryptophan) • Peptide derived: Angiotensin, Bradykinin • Lipid derived: Prostaglandins, Leukotrienes, Interleukins, Platelet Activating Factor, etc. FUNCTIONS • Physiological • Pathophysiological (Reaction to injuries) • Transmission and Modulation AMINE AUTACOIDS • DERIVED FROM NATURAL AMINO ACIDS • HISTAMINE AND SEROTONIN are the major autacoids in this class HISTAMINE NH2 5 4 1 3 N N H 2 Histamine INTRODUCTION • Imidazole ethylamine • Formed from the amino acid Histidine • Important inflammatory mediator • Potent biogenic amine and plays an important role in inflammation, anaphylaxis, allergies, gastric acid secretion and drug reaction • As part of an immune response to foreign pathogens, its produced by Basophils and mast cells found in nearby connective tissues. SITES OF HISTAMINE RELEASE 1) Mast cell site: Pulmonary tissue (mucosa of bronchial tree) • Skin • GIT(intestinal mucosa) • Conc. Of histamine is particularly high in these tissues 2) Non-mast cell sites: • CNS (neurons) • Epidermis of skin. • GIT(gastric cells) • Cells in regenerating or rapidly growing tissues • Basophils (in the blood) MECHANISM OF RELEASE • Histamine held by an acidic protein and heparin within intracellular granules → Granules extrude by exocytosis → Na+ gets exchanged for histamine • Substances released during IgG or lgM immunoreactions release histamine from the mast cells & basophil. • Chemical