Antihistamines on Zirchrom®-PS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 2. 2012 AGS Beers Criteria for Potentially
Table 2. 2012 AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults Strength of Organ System/ Recommendat Quality of Recomm Therapeutic Category/Drug(s) Rationale ion Evidence endation References Anticholinergics (excludes TCAs) First-generation antihistamines Highly anticholinergic; Avoid Hydroxyzin Strong Agostini 2001 (as single agent or as part of clearance reduced with e and Boustani 2007 combination products) advanced age, and promethazi Guaiana 2010 Brompheniramine tolerance develops ne: high; Han 2001 Carbinoxamine when used as hypnotic; All others: Rudolph 2008 Chlorpheniramine increased risk of moderate Clemastine confusion, dry mouth, Cyproheptadine constipation, and other Dexbrompheniramine anticholinergic Dexchlorpheniramine effects/toxicity. Diphenhydramine (oral) Doxylamine Use of diphenhydramine in Hydroxyzine special situations such Promethazine as acute treatment of Triprolidine severe allergic reaction may be appropriate. Antiparkinson agents Not recommended for Avoid Moderate Strong Rudolph 2008 Benztropine (oral) prevention of Trihexyphenidyl extrapyramidal symptoms with antipsychotics; more effective agents available for treatment of Parkinson disease. Antispasmodics Highly anticholinergic, Avoid Moderate Strong Lechevallier- Belladonna alkaloids uncertain except in Michel 2005 Clidinium-chlordiazepoxide effectiveness. short-term Rudolph 2008 Dicyclomine palliative Hyoscyamine care to Propantheline decrease Scopolamine oral secretions. Antithrombotics Dipyridamole, oral short-acting* May -

Diphenhydramine Hydrochloride (CASRN 147-24-0) in F344/N Rats
NATIONAL TOXICOLOGY PROGRAM Technical Report Series No. 355 TOXICOLOGY AND CARCINOGENESIS STUDIES OF DIPHENHYDRAMINE HYDROCHLORIDE (CAS NO. 147-24-0) IN F344/N RATS AND B6C3F1 MICE (FEED STUDIES) LJ.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health NTP ‘TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF DIPHENHYDRAMINE HYDROCHLORIDE (CAS NO. 147-24-0) IN F344/N RATS AND B6C3F1 MICE (FEED STUDIES) R. Melnick, Ph.D., Study Scientist NATIONAL TOXICOLOGY PROGRAM P.O. Box 12233 Research Triangle Park, NC 27709 September 1989 NTP TR 355 NIH Publication No. 89-2810 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health CONTENTS PAGE ABSTRACT ................................................................ 3 EXPLANATION OF LEVELS OF EVIDENCE OF CARCINOGENIC ACTIVITY .................. 6 CONTRIBUTORS ............................................................ 7 PEERREVIEWPANEL ........................................................ 8 SUMMARY OF PEER REVIEW COMMENTS ......................................... 9 I. INTRODUCTION ........................................................ 11 I1. MATERIALS AND METHODS .............................................. 21 III. RESULTS ............................................................. 35 RATS ............................................................. 36 MICE ............................................................. 45 GENETIC TOXICOLOGY ............................................... 53 IV. -

MSM Cross Reference Antihistamine Decongestant 20100701 Final Posted
MISSISSIPPI DIVISION OF MEDICAID Antihistamine/Decongestant Product and Active Ingredient Cross-Reference List The agents listed below are the antihistamine/decongestant drug products listed in the Mississippi Medicaid Preferred Drug List (PDL). This is a cross-reference between the drug product name and its active ingredients to reference the antihistamine/decongestant portion of the PDL. For more information concerning the PDL, including non- preferred agents, the OTC formulary, and other specifics, please visit our website at www.medicaid.ms.gov. List Effective 07/16/10 Therapeutic Class Active Ingredients Preferred Non-Preferred ANTIHISTAMINES - 1ST GENERATION BROMPHENIRAMINE MALEATE BPM BROMAX BROMPHENIRAMINE MALEATE J-TAN PD BROMSPIRO LODRANE 24 LOHIST 12HR VAZOL BROMPHENIRAMINE TANNATE BROMPHENIRAMINE TANNATE J-TAN P-TEX BROMPHENIRAMINE/DIPHENHYDRAM ALA-HIST CARBINOXAMINE MALEATE CARBINOXAMINE MALEATE PALGIC CHLORPHENIRAMINE MALEATE CHLORPHENIRAMINE MALEATE CPM 12 CHLORPHENIRAMINE TANNATE ED CHLORPED ED-CHLOR-TAN MYCI CHLOR-TAN MYCI CHLORPED PEDIAPHYL TANAHIST-PD CLEMASTINE FUMARATE CLEMASTINE FUMARATE CYPROHEPTADINE HCL CYPROHEPTADINE HCL DEXCHLORPHENIRAMINE MALEATE DEXCHLORPHENIRAMINE MALEATE DIPHENHYDRAMINE HCL ALLERGY MEDICINE ALLERGY RELIEF BANOPHEN BENADRYL BENADRYL ALLERGY CHILDREN'S ALLERGY CHILDREN'S COLD & ALLERGY COMPLETE ALLERGY DIPHEDRYL DIPHENDRYL DIPHENHIST DIPHENHYDRAMINE HCL DYTUSS GENAHIST HYDRAMINE MEDI-PHEDRYL PHARBEDRYL Q-DRYL QUENALIN SILADRYL SILPHEN DIPHENHYDRAMINE TANNATE DIPHENMAX DOXYLAMINE SUCCINATE -

2-Bromopyridine Safety Data Sheet Jubilant Ingrevia Limited
2-Bromopyridine Safety Data Sheet According to the federal final rule of hazard communication revised on 2012 (HazCom 2012) Date of Compilation : July 03 ’ 2019 Date of Revision : February 09 ’ 2021 Revision due date : January 2024 Revision Number : 01 Version Name : 0034Gj Ghs01 Div.3 sds 2-Bromopyridine Supersedes date : July 03 ’ 2019 Supersedes version : 0034Gj Ghs00 Div.3 sds 2-Bromopyridine Jubilant Ingrevia Limited Page 1 of 9 2-Bromopyridine Safety Data Sheet According to the federal final rule of hazard communication revised on 2012 (HazCom 2012) SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier PRODUCT NAME : 2-Bromopyridine CAS RN : 109-04-6 EC# : 203-641-6 SYNONYMS : 2-Pyridyl bromide, Pyridine, 2-bromo-, beta-Bromopyridine, o-Bromopyridine SYSTEMATIC NAME : 2-Bromopyridine, -Pyridine, 2-bromo- MOLECULAR FORMULA : C5H4BrN STRUCTURAL FORMULA N Br 1.2. Relevant identified uses of the substance or mixture and uses advised against 1.2.1. Relevant identified uses 2-Bromopyridine is used as an intermediate in the pharmaceutical industry for the manufacture of Atazanavir (an antiretroviral drug), Carbinoxamine, Chloropyramine, triprolidine (antihistamine drugs), Disopyramide Phosphate (an antiarrythmic drug), Mefloquine (antimalarial drug), Pipradrol (mild CNS stimulant) etc. Uses advised against: None 1.3. Details of the supplier of the safety data sheet Jubilant Ingrevia Limited REGISTERED & FACTORY OFFICE: Jubilant Ingrevia Limited Bhartiagram, Gajraula , District: Amroha, Uttar Pradesh-244223, India PHONE NO: +91-5924-252353 to 252360 Contact Department-Safety: Ext. 7424 , FAX NO : +91-5924-252352 HEAD OFFICE: Jubilant Ingrevia Limited, Plot 1-A, Sector 16-A,Institutional Area, Noida, Uttar Pradesh, 201301 - India T +91-120-4361000 - F +91-120-4234881 / 84 / 85 / 87 / 95 / 96 [email protected] -www.jubilantingrevia.com 1.4. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Methapyrilene Hydrochloride (CAS No
National Toxicology Program Toxicity Report Series Number 46 NTP Technical Report on the Hepatotoxicity Studies of the Liver Carcinogen Methapyrilene Hydrochloride (CAS No. 135-23-9) Administered in Feed to Male F344/N Rats July 2000 U.S. Department of Health and Human Services Public Health Service National Institutes of Health FOREWORD The National Toxicology Program (NTP) is made up of four charter agencies of the U.S. Department of Health and Human Services (DHHS): the National Cancer Institute (NCI), National Institutes of Health; the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health; the National Center for Toxicological Research (NCTR), Food and Drug Administration; and the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. In July 1981, the Carcinogenesis Bioassay Testing Program, NCI, was transferred to the NIEHS. The NTP coordinates the relevant programs, staff, and resources from these Public Health Service agencies relating to basic and applied research and to biological assay development and validation. The NTP develops, evaluates, and disseminates scientific information about potentially toxic and hazardous chemicals. This knowledge is used for protecting the health of the American people and for the primary prevention of disease. The studies described in this Toxicity Study Report were performed under the direction of the NIEHS and were conducted in compliance with NTP laboratory health and safety requirements and must meet or exceed all applicable federal, state, and local health and safety regulations. Animal care and use were in accordance with the Public Health Service Policy on Humane Care and Use of Animals. -

A Comparison of Drug Use in Driver Fatalities and Similarly Exposed Drivers
DOT HS-802 488 A COMPARISON OF DRUG USE IN DRIVER FATALITIES AND SIMILARLY EXPOSED DRIVERS Contract No. DOT-HS-4-00941 July 1977 Final Report PREPARED FOR: U.S. DEPARTMENT OF TRANSPORTATION NATIONAL HIGHWAY TRAFFIC SAFETY ADMINISTRATION WASHINGTON, D.C. 20590 Document is available to the public through the National Technical Information Service, Springfield, Virginia 22161 This document is disseminated under the sponsorship of the Department of Transportation in the interest of information exchange. The United States Govern ment assumes no liability for its contents or use thereof. Technical Report Documentation Page 1. Report No. 2. Goeemm.nt Accession No. 3. Recipient's Catalog No. DOT HS-802 488 -^. 1. Title and Subtitle 5. Report Date July 1977 A COMPARISON OF DRU^ USE IN DRIVER FATALITIES 6. Performing Organization Code AND SIMILARLY EXPOSED DRIVERS . B. Performing Organization Report No. 7. Authorrs) Robert R. Blackburn, Edward J. Woodhouse 3963-D 9. Performing Organization Name and Address 10. Worb Unit No. (TRAIS) Midwest Research Institute I). Contract at Grant No. 425 Volker Boulevard DOT-HS-4-00941 Kansas City, Missouri 64110 13. Type of Report and Period Covered 12. Sponsoring Agency Name and Address 28 June 1974-25 March 1977 i U.S. Department of Transportation National Highway Traffic Safety Administration Final Report Washington, D.C. 20590 14. Sponsoring Agency Code 15. supplementary Notes 16. Abstract Crash information, urine, blood and bile samples from 900 fatally injured drivers were collected by medical examiners in 22 areas of the country. Ran domly selected living drivers were interviewed at times and places of recent fatal crashes in Dallas, Texas, and Memphis, Tennessee and breath, urine, and blood samples were obtained. -

Medicines Classification Committee
Medicines Classification Committee Meeting date 1 May 2017 58th Meeting Title Reclassification of Sedating Antihistamines Medsafe Pharmacovigilance Submitted by Paper type For decision Team Proposal for The Medicines Adverse Reactions Committee (MARC) recommended that the reclassification to committee consider reclassifying sedating antihistamines to prescription prescription medicines when used in children under 6 years of age for the treatment of medicine for some nausea and vomiting and travel sickness [exact wording to be determined by indications the committee]. Reason for The purpose of this document is to provide the committee with an overview submission of the information provided to the MARC about safety concerns associated with sedating antihistamines and reasons for recommendations. Associated March 2013 Children and Sedating Antihistamines Prescriber Update articles February 2010 Cough and cold medicines clarification – antihistamines Medsafe website Safety information: Use of cough and cold medicines in children – new advice Medicines for Alimemazine Diphenhydramine consideration Brompheniramine Doxylamine Chlorpheniramine Meclozine Cyclizine Promethazine Dexchlorpheniramine New Zealand Some oral sedating antihistamines available without exposure to a prescription (pharmacist-only and pharmacy only), sedating therefore usage data is not easily available. antihistamines Table of Contents 1.0 PURPOSE ...................................................................................................................................... -
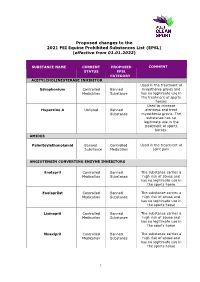
Proposed Changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (Effective from 01.01.2022)
Proposed changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (effective from 01.01.2022) SUBSTANCE NAME CURRENT PROPOSED COMMENT STATUS EPSL CATEGORY ACETYLCHOLINESTERASE INHIBITOR Used in the treatment of Edrophonium Controlled Banned myasthenia gravis and Medication Substance has no legitimate use in the treatment of sports horses Used to increase Huperzine A Unlisted Banned alertness and treat Substance myasthenia gravis. The substance has no legitimate use in the treatment of sports horses. AMIDES Palmitoylethanolamid Banned Controlled Used in the treatment of Substance Medication joint pain ANGIOTENSIN CONVERTING ENZYME INHIBITORS Enalapril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Enalaprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Lisinopril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Moexipril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse 1 Perindoprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse ANTIHISTAMINES Antazoline Controlled Banned The substance has no Medication Substance legitimate use in the sports horse Azatadine Controlled Banned The substance has Medication Substance sedative effects -
![With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S](https://docslib.b-cdn.net/cover/2862/with-3h-mepyramine-trieyclic-antidepressants-antihistamine-neurotransmitter-amitriptyline-vinh-tan-tran-raymond-s-1512862.webp)
With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S
Proc. Nati. Acad. Sci. USA Vol. 75, No. 12, pp. 6290-6294,, December 1978 Neurobiology Histamine H1 receptors identified in mammalian brain membranes with [3H]mepyramine (trieyclic antidepressants/antihistamine/neurotransmitter/amitriptyline) VINH TAN TRAN, RAYMOND S. L. CHANG, AND SOLOMON H. SNYDER* Departments of Pharmacology and Experimental Therapeutics, and Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205 Communicated by Julius Axelrod, August 30,1978 ABSTRACT The antihistamine [3H mepyramine binds to Male Sprague-Dawley rats (150-200 g) were killed by cer- HI histamine receptors in mammalian brain membranes. vical dislocation, their brains were rapidly removed and ho- Potencies of H1 antihistamines at the binding sites correlate mogenized with a Polytron for 30 min (setting 5) in 30 vol of with their pharmacological antihistamine effects in the guinea pig ileum. Specific [3Himepyramine binding is saturable with ice-cold Na/K phosphate buffer (50 mM, pH 7.5), and the a dissociation constant of about 4 nM in both equilibrium and suspension was centrifuged (50,000 X g for 10 min). The pellet kinetic experiments and a density of 10pmolper gram ofwhole was resuspended in the same volume of fresh buffer and cen- brain. Some tricyclic antidepressants are potent inhibitors of trifuged, and the final pellet was resuspended in the original secific [3Hmepamine binding. Regional variations of volume of ice-cold buffer by Polytron homogenization. Calf [3Hjmepyramine ing do not correlate with variations in brains were obtained from a local abattoir within 2 hr after the endogeneous histamine and histidine decarboxylase activity. death of the animals and transferred to the laboratory in ice- Histamine is a neurotransmitter candidate in mammalian brain cold saline. -

LITERATURE REVIEW Drug Review
LITERATURE REVIEW Drug Review Document ID: N04-023 Author: Julie Qidwai Date: December 2004 National Advanced Driving Simulator 2401 Oakdale Blvd. Iowa City, IA 52242-5003 Fax (319) 335-4658 TABLE OF CONTENTS 1 Introduction ............................................................................................................................ 1 2 Alprazolam.............................................................................................................................. 1 3 Amitriptyline........................................................................................................................... 2 4 Biperiden................................................................................................................................. 2 5 Brompheniramine .................................................................................................................. 2 6 Butorphanol ............................................................................................................................ 2 7 Cetirizine................................................................................................................................. 3 8 Chloroquine ............................................................................................................................ 3 9 Chlorpheniramine .................................................................................................................. 4 10 Clemastine.............................................................................................................................. -

A Unique Serotonin Receptor in Choroid Plexus Is Linked To
Proc. Natl. Acad. Sci. USA Vol. 83, pp. 4086-4088, June 1986 Neurobiology A unique serotonin receptor in choroid plexus is linked to phosphatidylinositol turnover (serotonin 5-HTjc site/serotonin 5-HT2 site/serotonin antagonists/cerebrospinal fluid/phosphoinositide hydrolysis) P. JEFFREY CONN*, ELAINE SANDERS-BUSH*t, BETH J. HOFFMANt, AND PAUL R. HARTIGt *Tennessee Neuropsychiatric Institute and Department of Pharmacology, Vanderbilt University School of Medicine, Nashville, TN 37232; and tDepartment of Biology, Johns Hopkins University, Baltimore, MD 21218 Communicated by Saul Roseman, January 23, 1986 ABSTRACT A novel serotonergic binding site, the 5-HTlc (11). These findings have been confirmed and extended in site, has been characterized recently in choroid plexus and brain (12-14) and other tissues (15-17). Serotonin-stimulated several brain regions. The biochemical and physiological roles phosphatidylinositol turnover in cerebral cortex is not sec- ofthis site have not been previously described. In this report we ondary to release of another neurotransmitter or of arachi- show that serotonin (5-hydroxytryptamine, 5-HT) stimulates donic acid (18), suggesting that the 5-HT2 receptor is directly phosphatidylinositol turnover in rat choroid plexus. The phar- coupled to phosphatidylinositol turnover. In the present macology of serotonin-stimulated phosphatidylinositol hydrol- studies, we have examined the effect of serotonin and ysis in choroid plexus was compared to the pharmacology in serotonin antagonists upon phosphatidylinositol turnover in cerebral cortex, where this response is mediated by the rat choroid plexus. The pharmacology of the response in serotonin 5-HT2 receptor. Serotonin increased phosphatidyl- choroid plexus was compared with that in cerebral cortex and inositol turnover in choroid plexus by 6-fold and in cerebral with the pharmacology ofthe 5-HT1c and 5-HT2 binding sites.