The Evolutionary History of Ehrhartoideae, Oryzeae, and Oryza
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -

Improved Conservation Plant Materials Released by NRCS and Cooperators Through December 2014
Natural Resources Conservation Service Improved Conservation Plant Materials Released by Plant Materials Program NRCS and Cooperators through December 2014 Page intentionally left blank. Natural Resources Conservation Service Plant Materials Program Improved Conservation Plant Materials Released by NRCS and Cooperators Through December 2014 Norman A. Berg Plant Materials Center 8791 Beaver Dam Road Building 509, BARC-East Beltsville, Maryland 20705 U.S.A. Phone: (301) 504-8175 prepared by: Julie A. DePue Data Manager/Secretary [email protected] John M. Englert Plant Materials Program Leader [email protected] January 2015 Visit our Website: http://Plant-Materials.nrcs.usda.gov TABLE OF CONTENTS Topics Page Introduction ...........................................................................................................................................................1 Types of Plant Materials Releases ........................................................................................................................2 Sources of Plant Materials ....................................................................................................................................3 NRCS Conservation Plants Released in 2013 and 2014 .......................................................................................4 Complete Listing of Conservation Plants Released through December 2014 ......................................................6 Grasses ......................................................................................................................................................8 -

Aquatic Macrophytes of Northeastern Brazil: Checklist, Richness
Check List 9(2): 298–312, 2013 © 2013 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution Aquatic macrophytes of Northeastern Brazil: Checklist, PECIES S richness, distribution and life forms OF Edson Gomes de Moura-Júnior 1, Liliane Ferreira Lima 1, Simone Santos Lira Silva 2, Raíssa Maria ISTS 3 4 5* 4 L Sampaio de Paiva , Fernando Alves Ferreira , Carmen Silvia Zickel and Arnildo Pott 1 Graduate student (PhD) in Plant Biology, Universidade Federal de Minas Gerais, Biology Department. Av. Antônio Carlos, 6627. CEP 31270-901. Belo Horizonte, MG, Brazil. 2 Graduate student (PhD) in Botany, Universidade Federal Rural de Pernambuco, Biology Department. Av. Dom Manoel de Medeiros, s/n°, Dois Irmãos. CEP 52171-900. Recife, PE, Brazil. 3 Graduate student (MSc) in Natural Resource, Universidade Federal de Roraima, Biology Department. Av. Capitão Ene Garcez, 2413, Aeroporto, Boa Vista. CEP 69304-000. Roraima, RR, Brazil. 4 Universidade Federal do Mato Grosso do Sul, Program in Plant Biology, Center for Biological Sciences and Health, Biology Department. Cidade Universitária, s/n - CEP 79070-900. Campo Grande, MS, Brazil. 5 Universidade Federal Rural de Pernambuco, Program in Botany, Biology Department. Av. Dom Manoel de Medeiros, s/n°, Dois Irmãos. CEP 52171-900. Recife, PE, Brazil. * Corresponding Author. E-mail: [email protected] Abstract: checklist of aquaticAquatic macrophytesplants have great occurring influence in the on northeastern the structure region and dynamics of Brazil throughof aquatic a bibliographic ecosystems, thereby search. Wecontributing recorded aconsiderably total of 412 tospecies, biodiversity. 217 genera In Brazil, and 72 knowledge families. -

Types of American Grasses
z LIBRARY OF Si AS-HITCHCOCK AND AGNES'CHASE 4: SMITHSONIAN INSTITUTION UNITED STATES NATIONAL MUSEUM oL TiiC. CONTRIBUTIONS FROM THE United States National Herbarium Volume XII, Part 3 TXE&3 OF AMERICAN GRASSES . / A STUDY OF THE AMERICAN SPECIES OF GRASSES DESCRIBED BY LINNAEUS, GRONOVIUS, SLOANE, SWARTZ, AND MICHAUX By A. S. HITCHCOCK z rit erV ^-C?^ 1 " WASHINGTON GOVERNMENT PRINTING OFFICE 1908 BULLETIN OF THE UNITED STATES NATIONAL MUSEUM Issued June 18, 1908 ii PREFACE The accompanying paper, by Prof. A. S. Hitchcock, Systematic Agrostologist of the United States Department of Agriculture, u entitled Types of American grasses: a study of the American species of grasses described by Linnaeus, Gronovius, Sloane, Swartz, and Michaux," is an important contribution to our knowledge of American grasses. It is regarded as of fundamental importance in the critical sys- tematic investigation of any group of plants that the identity of the species described by earlier authors be determined with certainty. Often this identification can be made only by examining the type specimen, the original description being inconclusive. Under the American code of botanical nomenclature, which has been followed by the author of this paper, "the nomenclatorial t}rpe of a species or subspecies is the specimen to which the describer originally applied the name in publication." The procedure indicated by the American code, namely, to appeal to the type specimen when the original description is insufficient to identify the species, has been much misunderstood by European botanists. It has been taken to mean, in the case of the Linnsean herbarium, for example, that a specimen in that herbarium bearing the same name as a species described by Linnaeus in his Species Plantarum must be taken as the type of that species regardless of all other considerations. -

Vascular Plants of Williamson County Leersia Hexandra Swartz
Vascular Plants of Williamson County Leersia hexandra − CLUBHEAD CUTGRASS [Poaceae] Leersia hexandra Swartz, CLUBHEAD CUTGRASS. Aquatic perennial herb, clonal, with submersed, short-lived rhizomes and stolon-bearing, fibrous-rooted, not rosetted, shoots in sparse clones with stolons arising from mother plant, mother plant rooted from short rhizome having several emergent shoots, stolons submersed and horizontal initially with 1 emergent shoot at a node, in range to 35 cm tall; flowering shoot with several basal leaves including at least 2 with photosynthetic blades and ca. 4 cauline leaves, axes with hairs at nodes, conspicuously scabrous, shoot unbranched with terminal inflorescence or with an ascending lateral shoot from an axillary bud above midplant; rhizomes poorly defined with many adventitious roots; stolons (termed rhizomes by other authors) with long, slender internodes, to 30 × ca. 0.7 mm, with 1−3 adventitious roots per node with or without an emergent shoot. Stems: cylindric, hidden by sheaths, internodes smooth, nodes purple- red, appearing channeled to flattened, to 2 mm diameter, having dense, stiff, appressed downward-pointing hairs (incl. basal of leaf sheath) with hirsute hairs to 1 mm long. Leaves: alternate distichous, simple with sheath; prophyll at base of emergent shoot of mother plant, to 10 mm long, membranous, light red-purple, appearing open, conspicuously 2-keeled with wide membranous margins, with sparse minute teeth along keels; sheath open but tightly sealed around stems, sheaths often with sparse, minute -

Ehrharta Calycina
Information on measures and related costs in relation to species considered for inclusion on the Union list: Ehrharta calycina This note has been drafted by IUCN within the framework of the contract No 07.0202/2017/763436/SER/ENV.D2 “Technical and Scientific support in relation to the Implementation of Regulation 1143/2014 on Invasive Alien Species”. The information and views set out in this note do not necessarily reflect the official opinion of the Commission, or IUCN. The Commission does not guarantee the accuracy of the data included in this note. Neither the Commission nor IUCN or any person acting on the Commission’s behalf, including any authors or contributors of the notes themselves, may be held responsible for the use which may be made of the information contained therein. Reproduction is authorised provided the source is acknowledged. This document shall be cited as: Visser, V. 2018. Information on measures and related costs in relation to species considered for inclusion on the Union list: Ehrharta calycina. Technical note prepared by IUCN for the European Commission. Date of completion: 25/10/2018 Comments which could support improvement of this document are welcome. Please send your comments by e-mail to [email protected]. Species (scientific name) Ehrharta calycina Sm. Pl. Ic. Ined. t. 33. Species (common name) Perennial veldt grass, purple veldt grass, veldt grass, common ehrharta, gewone ehrharta (Afrikaans), rooisaadgras (Afrikaans). Author(s) Vernon Visser, African Climate & Development Institute Date Completed 25/10/2018 Reviewer Courtenay A. Ray, Arizona State University Summary Highlight of measures that provide the most cost-effective options to prevent the introduction, achieve early detection, rapidly eradicate and manage the species, including significant gaps in information or knowledge to identify cost-effective measures. -

27April12acquatic Plants
International Plant Protection Convention Protecting the world’s plant resources from pests 01 2012 ENG Aquatic plants their uses and risks Implementation Review and Support System Support and Review Implementation A review of the global status of aquatic plants Aquatic plants their uses and risks A review of the global status of aquatic plants Ryan M. Wersal, Ph.D. & John D. Madsen, Ph.D. i The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of speciic companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned.All rights reserved. FAO encourages reproduction and dissemination of material in this information product. Non-commercial uses will be authorized free of charge, upon request. Reproduction for resale or other commercial purposes, including educational purposes, may incur fees. Applications for permission to reproduce or disseminate FAO copyright materials, and all queries concerning rights and licences, should be addressed by e-mail to [email protected] or to the Chief, Publishing Policy and Support Branch, Ofice of Knowledge Exchange, -

Montaña De Oro Checklist-07Jun19
Checklist1 of Vascular Flora of Montaña de Oro State Park San Luis Obispo County, California (07 June 2019) David J. Keil Robert F. Hoover Herbarium Biological Sciences Department California Polytechnic State University San Luis Obispo, California Scientific Name Common Name Family Rare n Abronia latifolia yellow sand-verbena NYCTAGINACEAE v n Abronia maritima beach sand-verbena, red NYCTAGINACEAE 4.2 v sand-verbena n Abronia umbellata var. umbellata purple sand-verbena NYCTAGINACEAE v n Acer macrophyllum big-leaf maple SAPINDACEAE v n ❀ Achillea millefolium yarrow ASTERACEAE v n Acmispon brachycarpus shortpod deervetch FABACEAE v 1 Please notify the author of additions or corrections to this list ([email protected]). ❀ — See Wildflowers of San Luis Obispo, California, second edition (2018) for photograph. Most are illustrated in the first edition as well; old names for some species in square brackets. n — California native n1 — California native but planted at Montaña de Oro. i — exotic species, introduced to California, naturalized or waif. v — documented by one or more specimens (Consortium of California Herbaria record; specimen in OBI; or collection that has not yet been accessioned) o — observed during field surveys; no voucher specimen known R—California Rare Plant Rank Scientific Name Common Name Family Rare n ❀ Acmispon glaber var. glaber common deerweed FABACEAE v n Acmispon heermannii var. orbicularis woolly deer-vetch FABACEAE v n Acmispon junceus var. biolettii Biolett's rush deerweed FABACEAE v n Acmispon junceus var. junceus common rush deerweed FABACEAE v n Acmispon maritimus var. maritimus coastal deer-vetch FABACEAE v n Acmispon micranthus fishhook deervetch FABACEAE v n Acmispon parviflorus miniature deervetch FABACEAE o n ❀ Acmispon strigosus strigose deer-vetch FABACEAE v n Actaea rubra baneberry RANUNCULACEAE v n ❀ Adelinia grandis Pacific hound's tongue BORAGINACEAE v n ❀ Adenostoma fasciculatum var. -
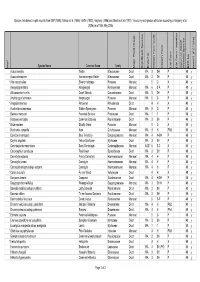
BFS048 Site Species List
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No kens01 (FCT23a) Wd? Acacia sessilis Wattle Mimosaceae Dicot WA 3 SH P 48 y Acacia stenoptera Narrow-winged Wattle Mimosaceae Dicot WA 3 SH P 48 y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 48 y Alexgeorgea nitens Alexgeorgea Restionaceae Monocot WA 6 S-R P 48 y Allocasuarina humilis Dwarf Sheoak Casuarinaceae Dicot WA 3 SH P 48 y Amphipogon turbinatus Amphipogon Poaceae Monocot WA 5 G P 48 y * Anagallis arvensis Pimpernel Primulaceae Dicot 4 H A 48 y Austrostipa compressa Golden Speargrass Poaceae Monocot WA 5 G P 48 y Banksia menziesii Firewood Banksia Proteaceae Dicot WA 1 T P 48 y Bossiaea eriocarpa Common Bossiaea Papilionaceae Dicot WA 3 SH P 48 y * Briza maxima Blowfly Grass Poaceae Monocot 5 G A 48 y Burchardia congesta Kara Colchicaceae Monocot WA 4 H PAB 48 y Calectasia narragara Blue Tinsel Lily Dasypogonaceae Monocot WA 4 H-SH P 48 y Calytrix angulata Yellow Starflower Myrtaceae Dicot WA 3 SH P 48 y Centrolepis drummondiana Sand Centrolepis Centrolepidaceae Monocot AUST 6 S-C A 48 y Conostephium pendulum Pearlflower Epacridaceae Dicot WA 3 SH P 48 y Conostylis aculeata Prickly Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis juncea Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis setigera subsp. -

Biodiversity and Ecology of Critically Endangered, Rûens Silcrete Renosterveld in the Buffeljagsrivier Area, Swellendam
Biodiversity and Ecology of Critically Endangered, Rûens Silcrete Renosterveld in the Buffeljagsrivier area, Swellendam by Johannes Philippus Groenewald Thesis presented in fulfilment of the requirements for the degree of Masters in Science in Conservation Ecology in the Faculty of AgriSciences at Stellenbosch University Supervisor: Prof. Michael J. Samways Co-supervisor: Dr. Ruan Veldtman December 2014 Stellenbosch University http://scholar.sun.ac.za Declaration I hereby declare that the work contained in this thesis, for the degree of Master of Science in Conservation Ecology, is my own work that have not been previously published in full or in part at any other University. All work that are not my own, are acknowledge in the thesis. ___________________ Date: ____________ Groenewald J.P. Copyright © 2014 Stellenbosch University All rights reserved ii Stellenbosch University http://scholar.sun.ac.za Acknowledgements Firstly I want to thank my supervisor Prof. M. J. Samways for his guidance and patience through the years and my co-supervisor Dr. R. Veldtman for his help the past few years. This project would not have been possible without the help of Prof. H. Geertsema, who helped me with the identification of the Lepidoptera and other insect caught in the study area. Also want to thank Dr. K. Oberlander for the help with the identification of the Oxalis species found in the study area and Flora Cameron from CREW with the identification of some of the special plants growing in the area. I further express my gratitude to Dr. Odette Curtis from the Overberg Renosterveld Project, who helped with the identification of the rare species found in the study area as well as information about grazing and burning of Renosterveld. -

Garden Escapes & Other Weeds in Bushland and Reserves a Responsible Gardening Guide for the Sydney Region
Garden Escapes & Other Weeds in Bushland and Reserves A responsible gardening guide for the Sydney Region Sydney Weeds Committees Sydney Central Sydney South West Sydney North Sydney West – Blue Mountains C O N T E N T S General Information 3 Vines & Scramblers 6 Ground Covers 20 Bulbous & Succulent Weeds 34 Grass Weeds 51 Shrub Weeds 57 Tree Weeds 64 Water Weeds 74 Help Protect Your Local Environment 77 Common Plant Parts 78 Bibliography 79 Plant Me Instead 80 Index & Acknowledments 82 Reprinted 2012- Updated in 2018 Booklet adapted and reproduced with permission of Great Lakes Council The Problem What is a weed? Plants escape from gardens in a WEEDS are plants that don’t belong variety of ways, but one main cause where they are. They can include of spread from gardens is by green plants from other countries but are also waste dumping in bushland and road sometimes from other parts of Australia. reserves. This practice is harmful to the Weeds can be harmful to human and bush for many reasons, such as: animals. They also affect the ecology and appearance of bushland areas and s introducing weeds (plant fragments, waterways. bulbs, roots, tubers, seeds, spores) Weeds often grow faster than s smothering native plants native plants and out-compete them to become dominant in natural areas. The s changing the soil and ideal growing natural pests or diseases that would conditions for native plants otherwise control their growth are lacking s increasing fi re risk by increasing as the plants have been introduced from fuel loads. somewhere else. -

Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(S): Grass Phylogeny Working Group, Nigel P
Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(s): Grass Phylogeny Working Group, Nigel P. Barker, Lynn G. Clark, Jerrold I. Davis, Melvin R. Duvall, Gerald F. Guala, Catherine Hsiao, Elizabeth A. Kellogg, H. Peter Linder Source: Annals of the Missouri Botanical Garden, Vol. 88, No. 3 (Summer, 2001), pp. 373-457 Published by: Missouri Botanical Garden Press Stable URL: http://www.jstor.org/stable/3298585 Accessed: 06/10/2008 11:05 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=mobot. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit organization founded in 1995 to build trusted digital archives for scholarship. We work with the scholarly community to preserve their work and the materials they rely upon, and to build a common research platform that promotes the discovery and use of these resources. For more information about JSTOR, please contact [email protected].