Fundamentals of Glycan Structure 2
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Electronic Supplementary Information
Electronic Supplementary Material (ESI) for Chemical Science. This journal is © The Royal Society of Chemistry 2019 Electronic Supplementary Information Poly(ionic liquid)s as a Distinct Receptor Material to Create Highly- Integrated Sensing Platform for Efficiently Identifying a Myriad of Saccharides Wanlin Zhang, Yao Li, Yun Liang, Ning Gao, Chengcheng Liu, Shiqiang Wang, Xianpeng Yin, and Guangtao Li* *Corresponding authors: Guangtao Li ([email protected]) S1 Contents 1. Experimental Section (Page S4-S6) Materials and Characterization (Page S4) Experimental Details (Page S4-S6) 2. Figures and Tables (Page S7-S40) Fig. S1 SEM image of silica colloidal crystal spheres and PIL inverse opal spheres. (Page S7) Fig. S2 Adsorption isotherm of PIL inverse opal. (Page S7) Fig. S3 Dynamic mechanical analysis and thermal gravimetric analysis of PIL materials. (Page S7) Fig. S4 Chemical structures of 23 saccharides. (Page S8) Fig. S5 The counteranion exchange of PIL photonic spheres from Br- to DCA. (Page S9) Fig. S6 Reflection and emission spectra of spheres for saccharides. (Page S9) Table S1 The jack-knifed classification on single-sphere array for 23 saccharides. (Page S10) Fig. S7 Lower detection concentration at 10 mM of the single-sphere array. (Page S11) Fig. S8 Lower detection concentration at 1 mM of the single-sphere array. (Page S12) Fig. S9 PIL sphere exhibiting great pH robustness within the biological pH range. (Page S12) Fig. S10 Exploring the tolerance of PIL spheres to different conditions. (Page S13) Fig. S11 Exploring the reusability of PIL spheres. (Page S14) Fig. S12 Responses of spheres to sugar alcohols. (Page S15) Fig. -

Effect of Metal Chlorides on Glucose Mutarotation and Possible Implications on Humin Formation
Reaction Chemistry & Engineering Effect of Metal Chlorides on Glucose Mutarotation and Possible Implications on Humin Formation Journal: Reaction Chemistry & Engineering Manuscript ID RE-COM-10-2018-000233.R1 Article Type: Communication Date Submitted by the 28-Nov-2018 Author: Complete List of Authors: Ramesh, Pranav; Rutgers The State University of New Jersey, Chemical and Biochemical Engineering Kritikos, Athanasios; Rutgers The State University of New Jersey, Chemical and Biochemical Engineering Tsilomelekis, George; Rutgers The State University of New Jersey, Chemical and Biochemical Engineering Page 1 of 5 ReactionPlease doChemistry not adjust & Engineering margins Journal Name COMMUNICATION Effect of Metal Chlorides on Glucose Mutarotation and Possible Implications on Humin Formation a a a Received 00th January 20xx, Pranav Ramesh , Athanasios Kritikos and George Tsilomelekis * Accepted 00th January 20xx DOI: 10.1039/x0xx00000x www.rsc.org/ An in-situ Raman spectroscopic kinetic study of the glucose suggested that the possible changes in the anomeric mutarotation reaction is presented herein. The effect of metal equilibrium especially via the stabilization of the α-anomer of chlorides on the ease of ring opening process is discussed. It is glucose, might be responsible for improved selectivity towards 7, 8 shown that SnCl4 facilitates the mutarotation process towards the fructose . This also agrees with the concept of anomeric β-anomer extremely fast, while CrCl3 appears to promote the specificity of enzymes; for instance, immobilized D-glucose formation of the α-anomer of glucose. Infrared spectra of humins isomerase has shown ~40% and ~110% higher conversion rates prepared in different Lewis acids underscore the posibility of starting with α–D-glucose as compared to equilibrated glucose multiple reaction pathways. -

Structural Features
1 Structural features As defined by the International Union of Pure and Applied Chemistry gly- cans are structures of multiple monosaccharides linked through glycosidic bonds. The terms sugar and saccharide are synonyms, depending on your preference for Arabic (“sukkar”) or Greek (“sakkēaron”). Saccharide is the root for monosaccha- rides (a single carbohydrate unit), oligosaccharides (3 to 20 units) and polysac- charides (large polymers of more than 20 units). Carbohydrates follow the basic formula (CH2O)N>2. Glycolaldehyde (CH2O)2 would be the simplest member of the family if molecules of two C-atoms were not excluded from the biochemical repertoire. Glycolaldehyde has been found in space in cosmic dust surrounding star-forming regions of the Milky Way galaxy. Glycolaldehyde is a precursor of several organic molecules. For example, reaction of glycolaldehyde with propenal, another interstellar molecule, yields ribose, a carbohydrate that is also the backbone of nucleic acids. Figure 1 – The Rho Ophiuchi star-forming region is shown in infrared light as captured by NASA’s Wide-field Infrared Explorer. Glycolaldehyde was identified in the gas surrounding the star-forming region IRAS 16293-2422, which is is the red object in the centre of the marked square. This star-forming region is 26’000 light-years away from Earth. Glycolaldehyde can react with propenal to form ribose. Image source: www.eso.org/public/images/eso1234a/ Beginning the count at three carbon atoms, glyceraldehyde and dihydroxy- acetone share the common chemical formula (CH2O)3 and represent the smallest carbohydrates. As their names imply, glyceraldehyde has an aldehyde group (at C1) and dihydoxyacetone a carbonyl group (at C2). -

WO 2013/070444 Al 16 May 2013 (16.05.2013) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/070444 Al 16 May 2013 (16.05.2013) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A23G 4/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US20 12/062043 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 26 October 2012 (26.10.2012) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (26) Publication Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (30) Priority Data: ZM, ZW. 61/556,546 7 November 20 11 (07. 11.201 1) US (84) Designated States (unless otherwise indicated, for every (71) Applicant (for all designated States except US): WVI. kind of regional protection available): ARIPO (BW, GH, WRIGLEY JR. COMPANY [US/US]; 1132 Blackhawk GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, Street, Chicago, IL 60642 (US). -

Monosaccharides: a Tof-SIMS Reference Accession #: 01592, 01593, 01594, 01595, 01596, 01597, 01598, Spectra Database
Monosaccharides: A ToF-SIMS reference Accession #: 01592, 01593, 01594, 01595, 01596, 01597, 01598, spectra database. II. Positive polarity 01599, 01600, 01601, 01602, a) 01603, 01604, 01605, 01606, Laetitia Bernard, Rowena Crockett, and Maciej Kawecki 01607, 01608, 01609, 01610 Laboratory of Nanoscale Materials Science, Empa, CH-8600 Dübendorf, Switzerland Technique: SIMS (Received 20 August 2019; accepted 30 October 2019; published 3 December 2019) Host Material: Silicon (100) wafer The number of time-of-flight secondary ion mass spectrometry studies on biological tissues and Instrument: IONTOF TOF-SIMS.5 cells has strongly increased since the development of primary ion sources that allow not only ele- Major Species in Spectra: C, H, O, (N) mental but also molecular analysis. Substantial fragmentation during ionic bombardment results in a Minor Species in Spectra: Na, K large number of peaks, rendering data analysis complex. Complete and trustable sets of reference spectra for the main biological building blocks, i.e., amino acids, monosaccharides, fatty acids, and Published Spectra: 19 nucleotides, are required. This work aims to provide an accurate and extensive library of reference Spectra in Electronic Record: 19 + spectra for monosaccharides, measured with the Bi3 primary ion. Here (Paper II), the positive polar- Published Figures: 20 ity spectra and lists of associated characteristic fragments are presented. Published by the AVS. Spectral Category: Reference https://doi.org/10.1116/1.5125103 Keywords: ToF-SIMS, carbohydrate, sugar, monosaccharide, mass spectrometry, fragmentation INTRODUCTION Fluka and all others from Sigma Aldrich. Each powder was dissolved in freshly de-ionized H2O (resistivity >18.2 MΩ cm) Monosaccharides are the building blocks of structural polymers at a concentration of 0.1M. -

Food Carbohydrates: Monosaccharides and Oligosaccharides
Paper No. 01 Paper Title: Food Chemistry Module-04: Food carbohydrates: Monosaccharides and Oligosaccharides Monosaccharides The simplest form of carbohydrates is the monosaccharide. Monosaccharides are either aldoses or ketoses. Aldoses such as glucose consists of a carbon backbone and a carbonyl group (C=O) located at the end of the chain. Ketoses such as fructose consists of a carbon backbone with a carbonyl group located at any other carbon in the chain. The remaining carbon atoms are bound to hydroxyl groups (-OH). Monosaccharide classifications based on the number of carbons Number Category of Examples Name Carbons 4 Tetrose Erythrose, Threose 5 Pentose Arabinose, Ribose, Ribulose, Xylose, Xylulose, Lyxose Allose, Altrose, Fructose, Galactose, Glucose, Gulose, Idose, 6 Hexose Mannose, Sorbose, Talose, Tagatose 7 Heptose Sedoheptulose, Mannoheptulose Monosaccharides Three common sugars glucose, galactose and fructose share the same molecular formula: C6H12O6. Because of their six carbon atoms, each is a hexose. Although all three share the same molecular formula, the arrangement of atoms differs in each case. Substances such as these three, which have identical molecular formulas but different structural formulas, are known as structural isomers. Glucose "Blood sugar" is the immediate source of energy for cellular respiration. Glucose, which is also referred to as dextrose, is a moderately sweet sugar found in vegetables and fruit. When glucose is fermented by the enzyme zymase, in yeast, it results in the formation of carbon dioxide and ethyl alcohol. It is the basic structure to which all carbohydrates are reduced to in the end, for transport via the bloodstream and use by the cells of the body. -
![25 05.Html.Ppt [Read-Only]](https://docslib.b-cdn.net/cover/0806/25-05-html-ppt-read-only-1790806.webp)
25 05.Html.Ppt [Read-Only]
25.5 A Mnemonic for Carbohydrate Configurations The Eight D-Aldohexoses CH O H OH CH2OH The Eight D-Aldohexoses All CH O Altruists Gladly Make Gum In H OH Gallon CH2OH Tanks The Eight D-Aldohexoses All Allose CH O Altruists Altrose Gladly Glucose Make Mannose Gum Gulose In Idose H OH Gallon Galactose CH2OH Tanks Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose Gulose Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose Gulose H OH Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose Gulose HO H Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose Gulose H OH Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose H OH Gulose H OH Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose HO H Gulose H OH Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose Gulose HO H Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose H OH Gulose HO H Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose HO H Gulose HO H Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose H OH Gulose H OH Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose H OH Mannose H OH Gulose H OH Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose -

United States Patent (19 11) 4,312,979 Takemoto Et Al
United States Patent (19 11) 4,312,979 Takemoto et al. 45 Jan. 26, 1982 54 POLYSACCHARIDES CONTAINING 58) Field of Search ......................... 536/1, 18, 114, 4; ALLOSE 435/101 (75) Inventors: Hisao Takemoto; Tatsuo Igarashi, (56) References Cited both of Shin-Nanyo, Japan U.S. PATENT DOCUMENTS 73 Assignee: Toyo Soda Manufacturing Co., Ltd., 3,711,462 l/1973 Abdo et al. ............................. 536/1 Tokyo, Japan 4,186,025 l/1980 Kang et al. ............................. 536/1 Primary Examiner-Johnnie R. Brown (21) Appl. No.: 30,444 Attorney, Agent, or Firm-Scully, Scott, Murphy & Presser 22 Filed: Apr. 16, 1979 57 ABSTRACT 30 Foreign Application Priority Data A new polysaccharide including allose as a constituent Apr. 20, 1978 JP Japan .................................. 53.45918 sugar and further characterized by galactose as a major Dec. 5, 1978 JP Japan ................................ 53-149715 constituent sugar is described. The polysaccharide is produced extracellularly by cultivation of Pseudomonas 51) Int. Cl. ............................................... CO7H1/08 viscogena strains in nutrient medium. 52 U.S. C. ...... 0 a a 4 536/1; 435/72; 435/101; 536/114 6 Claims, 2 Drawing Figures U.S. Patent Jan. 26, 1982 Sheet 1 of 2 4,312,979 8 O O Sn Cd O ar - O - 3 CD won L Cd O CO ve O Cd Cd cN O O O N O O o O o O O. d o o O a. 3 c) do N. ud to at Y on 9 (%) AONWLLIWSNW U.S. Patent Jan. 26, 1982 Sheet 2 of 2 4,312,979 3 s 8 un co, t OO O) O S s S 8 & O O O O O r ONWSOS9W 4,312,979 1. -

Mutarotation
13 Mutarotation The α and β anomers are diastereomers of each other and usually have different specific rotations. A solution or liquid sample of a pure α anomer will rotate plane polarised light by a different amount and/or in the opposite direction than the pure β anomer of that compound. The optical rotation of the solution depends on the optical rotation of each anomer and their ratio in the solution. For example, if a solution of β-D-glucopyranose is dissolved in water, its specific optical rotation will be +18.7°. Over time, some of the β-D-glucopyranose will undergo mutarotation to become α-D-glucopyranose, which has an optical rotation of +112.2°. Thus the rotation of the solution will increase from +18.7° to an equilibrium value of +52.7° as some of the β form is converted to the α form. The equilibrium mixture is actually about 64% of β-D-glucopyranose and about 36% of α-D-glucopyranose, though there are also traces of the other forms including furanoses and open chained form. The α anomer is the major conformer, although somewhat controversially; this is due to the anomeric effect with the stabilization energy provided by n–σ* hyperconjugation. The observed rotation of the sample is the weighted sum of the optical rotation of each anomer weighted by the amount of that anomer present. 14 Therefore, one can use a polarimeter to measure the rotation of a sample and then calculate the ratio of the two anomers present from the enantiomeric excess, as long as one knows the rotation of each pure anomer. -

Thermodynamic and Kinetic Studies of Glucose Mutarotation by Using a Portable Personal Blood Glucose Meter Carlos Eduardo Perles* and Pedro Luiz Onófrio Volpe
Acta Chim. Slov. 2009, 56, 209–214 209 Short communication Thermodynamic and Kinetic Studies of Glucose Mutarotation by Using a Portable Personal Blood Glucose Meter Carlos Eduardo Perles* and Pedro Luiz Onófrio Volpe Instituto de Química, Universidade Estadual de Campinas – UNICAMP, CP. 6154 CEP. 13084-971, Campinas, SP – Brazil * Corresponding author: E-mail: [email protected] Phone: +55 (19) 3521-3091 Received: 28-08-2008 Dedicated to Professor Josef Barthel on the occasion of his 80th birthday Abstract A thermodynamic and kinetic study of the mutarotation reaction of D-glucose in aqueous solution was carried out using a portable personal blood glucose meter. This physical chemical experiment is proposed as an alternative to classical po- larimetry. The glucose meter allows the indirect monitoring of the mutarotation process in water, by using an enzymatic redox reaction. The test strips of the glucose meter contain glucose dehydrogenase which converts β-D-glucose into D- glucolactone. This reaction selectively converts glucose and generates an electrical current in the glucose meter which is proportional to the glucose concentration. This experiment allows the teacher to explore the kinetics and thermodynamics of the mutarotation of D-glucose and, moreover, the stereospecificity of enzymatic reactions. Keywords: Glucose, mutarotation, blood glucose meter 1. Introduction are present in a cyclic form in solution, with a very small amount of the acyclic form (∼0.01%)2, 3. The mutarotation Glucose is a polyhydroxy aldehyde belonging to the is a dynamic process resulting from a chemical reaction chemical class of carbohydrates. It is the most abundant that promotes the change between the forms α- and β-D- and most important carbohydrate in nature, since the me- glucose in solution, which has a pseudo-first order reac- tabolism of a majority of living species is based on the tion kinetics in aqueous solutions. -

Biochemistry Introductory Lecture Dr
Biochemistry Introductory lecture Dr. Munaf S. Daoud Carbohydrates (CHO) Definition: Aldehyde or Ketone derivatives of the higher polyhydric alcohols or compounds which yield these derivatives on hydrolysis. Classification: (mono, di, oligo, poly) saccharide. Monosaccharides: Can be classified as trioses, tetroses, pentoses, hexoses and heptoses depending upon the number of carbon atoms, and as aldoses or ketoses, depending upon whether they have an aldehyde or ketone group. Aldehyde (-CHO) Aldoses Ketone (-C=O) Ketoses Polysaccharides (glycans): Homopolysaccharides (homoglycans): e.g. starch, glycogen, inulin, cellulose, dextrins, dextrans. Heteropolysaccharides (heteroglycans): e.g. mucopolysaccharides (MPS) or glycosaminoglycans. Function of CHO: 1) Chief source of energy (immediate and stored energy). 2) Constituent of compound lipids and conjugated protein. 3) Structural element like cellulose. 4) Drugs like cardiac glycosides and antibodies. 5) Lactating mammary gland (Lactose in milk). 6) Synthesis of other substances like fatty acids, cholesterol, amino acids…etc. by their degradation products. 7) Constituent of mucopolysaccharides. 1 1) Stereo-isomerism Stereo-isomers: D-form, L-form 2) Optical isomers (optical activity) Enantiomers: dextrorotatory (d or + sign) Levorotatory (l or – sign) Racemic (d l) 3) Cyclic structures or open chain 4) Anomers and Anomeric carbon OH on carbon number 1, if below the plane then its -form, if above the plane then -form. Mutarotation: the changes of the initial optical rotation that takes place -
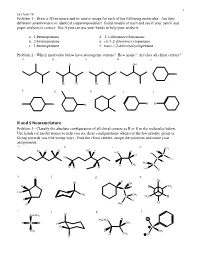
R and S Nomemclature Problem 3 - Classify the Absolute Configuration of All Chiral Centers As R Or S in the Molecules Below
1 Lecture 16 Problem 1 - Draw a 3D structure and its mirror image for each of the following molecules. Are they different (enantiomers) or identical (superimposable)? Build models of each and see if your pencil and paper analysis is correct. See if you can use your hands to help your analysis. a. 1-bromopentane d. 1,1-dibromocyclopentane b. 2-bromopentane e. cis-1,2-dibromocyclopentane c. 3-bromopentane f. trans-1,2-dibromocyclopentane Problem 2 - Which molecules below have stereogenic centers? How many? Are they all chiral centers? a. b. c. d. e. Cl OH Br Br Cl Br Br f. g. h. i. j. Br Br Br Br R and S Nomemclature Problem 3 - Classify the absolute configuration of all chiral centers as R or S in the molecules below. Use hands (or model atoms) to help you see these configurations whenever the low priority group is facing towards you (the wrong way). Find the chiral centers, assign the priorities and make your assignments. a. b. c. d. CH3 Cl H H H3C H H H H3C Br Cl C HO C CH3 I H H3C H CH3 C H H H H3C H e. O f. g. h. H Br Br OH H H H CH3 C H3C CH3 CH3 C H3C H H H H C H Cl 3 i. j. k. l. Cl CH2CH3 Br H3CH2C H C Br H CH3 Cl S CH3 D CH3 H CH3 O H 2 Lecture 16 Pi Bond Priority Problem 4 - Evaluate the order of priority in each part.