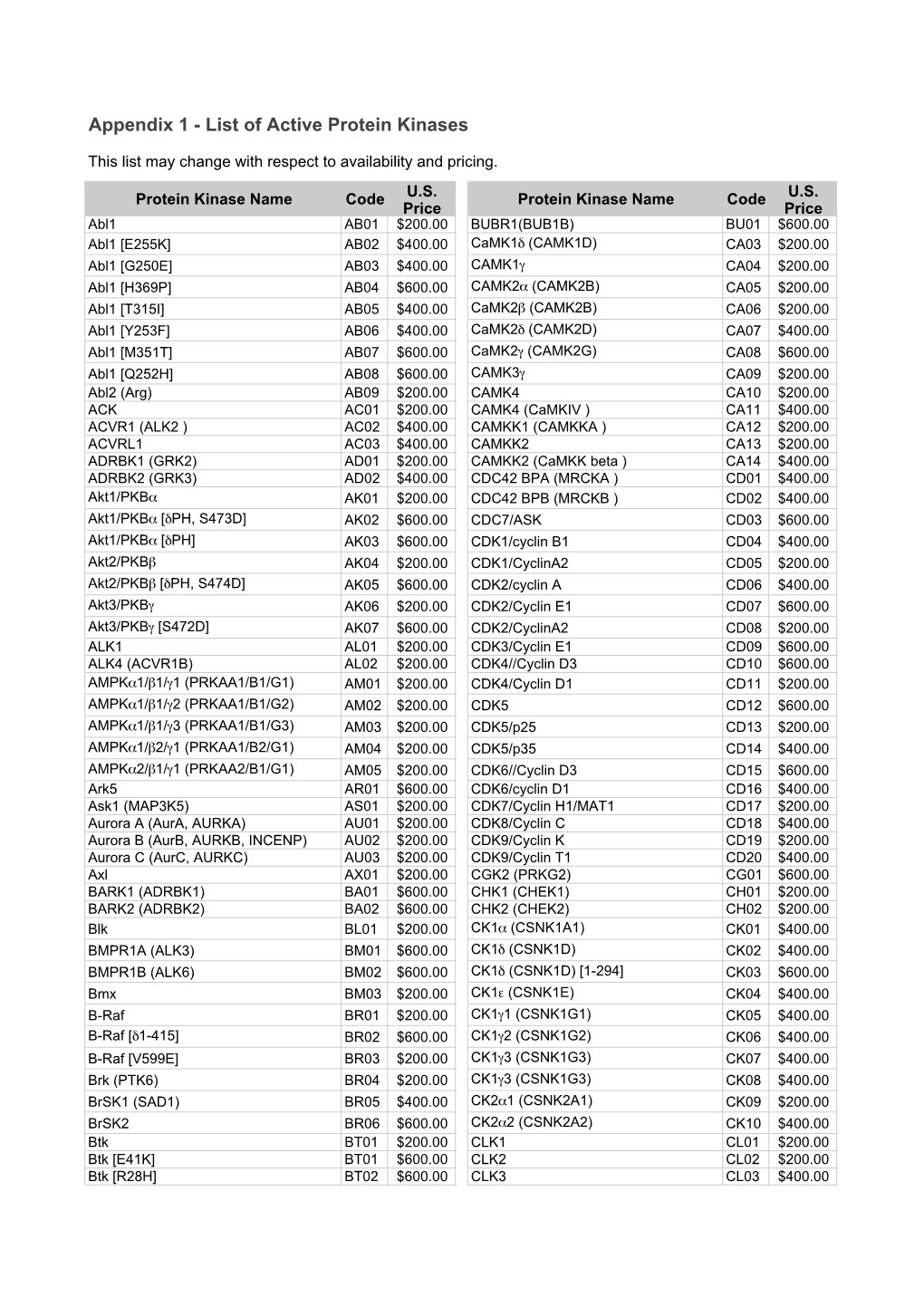
Download a Pdf with a List of Active Protein Kinases Available From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplemental Information to Mammadova-Bach Et Al., “Laminin Α1 Orchestrates VEGFA Functions in the Ecosystem of Colorectal Carcinogenesis”
Supplemental information to Mammadova-Bach et al., “Laminin α1 orchestrates VEGFA functions in the ecosystem of colorectal carcinogenesis” Supplemental material and methods Cloning of the villin-LMα1 vector The plasmid pBS-villin-promoter containing the 3.5 Kb of the murine villin promoter, the first non coding exon, 5.5 kb of the first intron and 15 nucleotides of the second villin exon, was generated by S. Robine (Institut Curie, Paris, France). The EcoRI site in the multi cloning site was destroyed by fill in ligation with T4 polymerase according to the manufacturer`s instructions (New England Biolabs, Ozyme, Saint Quentin en Yvelines, France). Site directed mutagenesis (GeneEditor in vitro Site-Directed Mutagenesis system, Promega, Charbonnières-les-Bains, France) was then used to introduce a BsiWI site before the start codon of the villin coding sequence using the 5’ phosphorylated primer: 5’CCTTCTCCTCTAGGCTCGCGTACGATGACGTCGGACTTGCGG3’. A double strand annealed oligonucleotide, 5’GGCCGGACGCGTGAATTCGTCGACGC3’ and 5’GGCCGCGTCGACGAATTCACGC GTCC3’ containing restriction site for MluI, EcoRI and SalI were inserted in the NotI site (present in the multi cloning site), generating the plasmid pBS-villin-promoter-MES. The SV40 polyA region of the pEGFP plasmid (Clontech, Ozyme, Saint Quentin Yvelines, France) was amplified by PCR using primers 5’GGCGCCTCTAGATCATAATCAGCCATA3’ and 5’GGCGCCCTTAAGATACATTGATGAGTT3’ before subcloning into the pGEMTeasy vector (Promega, Charbonnières-les-Bains, France). After EcoRI digestion, the SV40 polyA fragment was purified with the NucleoSpin Extract II kit (Machery-Nagel, Hoerdt, France) and then subcloned into the EcoRI site of the plasmid pBS-villin-promoter-MES. Site directed mutagenesis was used to introduce a BsiWI site (5’ phosphorylated AGCGCAGGGAGCGGCGGCCGTACGATGCGCGGCAGCGGCACG3’) before the initiation codon and a MluI site (5’ phosphorylated 1 CCCGGGCCTGAGCCCTAAACGCGTGCCAGCCTCTGCCCTTGG3’) after the stop codon in the full length cDNA coding for the mouse LMα1 in the pCIS vector (kindly provided by P. -

Gene Symbol Gene Description ACVR1B Activin a Receptor, Type IB
Table S1. Kinase clones included in human kinase cDNA library for yeast two-hybrid screening Gene Symbol Gene Description ACVR1B activin A receptor, type IB ADCK2 aarF domain containing kinase 2 ADCK4 aarF domain containing kinase 4 AGK multiple substrate lipid kinase;MULK AK1 adenylate kinase 1 AK3 adenylate kinase 3 like 1 AK3L1 adenylate kinase 3 ALDH18A1 aldehyde dehydrogenase 18 family, member A1;ALDH18A1 ALK anaplastic lymphoma kinase (Ki-1) ALPK1 alpha-kinase 1 ALPK2 alpha-kinase 2 AMHR2 anti-Mullerian hormone receptor, type II ARAF v-raf murine sarcoma 3611 viral oncogene homolog 1 ARSG arylsulfatase G;ARSG AURKB aurora kinase B AURKC aurora kinase C BCKDK branched chain alpha-ketoacid dehydrogenase kinase BMPR1A bone morphogenetic protein receptor, type IA BMPR2 bone morphogenetic protein receptor, type II (serine/threonine kinase) BRAF v-raf murine sarcoma viral oncogene homolog B1 BRD3 bromodomain containing 3 BRD4 bromodomain containing 4 BTK Bruton agammaglobulinemia tyrosine kinase BUB1 BUB1 budding uninhibited by benzimidazoles 1 homolog (yeast) BUB1B BUB1 budding uninhibited by benzimidazoles 1 homolog beta (yeast) C9orf98 chromosome 9 open reading frame 98;C9orf98 CABC1 chaperone, ABC1 activity of bc1 complex like (S. pombe) CALM1 calmodulin 1 (phosphorylase kinase, delta) CALM2 calmodulin 2 (phosphorylase kinase, delta) CALM3 calmodulin 3 (phosphorylase kinase, delta) CAMK1 calcium/calmodulin-dependent protein kinase I CAMK2A calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha CAMK2B calcium/calmodulin-dependent -

Brief Genetics Report Haplotype Structures and Large
Brief Genetics Report Haplotype Structures and Large-Scale Association Testing of the 5 AMP-Activated Protein Kinase Genes PRKAA2, PRKAB1, and PRKAB2 With Type 2 Diabetes Maria W. Sun,1,2 Jennifer Y. Lee,1,2 Paul I.W. de Bakker,1,2,3 Noe¨l P. Burtt,2 Peter Almgren,4 Lennart Råstam,5 Tiinamaija Tuomi,6 Daniel Gaudet,7 Mark J. Daly,2,8 Joel N. Hirschhorn,2,3,9 David Altshuler,1,2,3,8,10 Leif Groop,4,6 and Jose C. Florez1,2,8,10 AMP-activated protein kinase (AMPK) is a key molecular plasma glucose, or insulin sensitivity. Several nominal asso- regulator of cellular metabolism, and its activity is induced ciations of variants in PRKAA2 and PRKAB1 with BMI appear by both metformin and thiazolidinedione antidiabetic med- to be consistent with statistical noise. Diabetes 55:849–855, ications. It has therefore been proposed both as a putative 2006 agent in the pathophysiology of type 2 diabetes and as a valid target for therapeutic intervention. Thus, the genes that encode the various AMPK subunits are intriguing ype 2 diabetes arises from the complex interplay candidates for the inherited basis of type 2 diabetes. We therefore set out to test for the association of common of various pathophysiologic mechanisms involv- variants in the genes that encode three selected AMPK ing peripheral insulin resistance and relative subunits with type 2 diabetes and related phenotypes. Of Tinsulin insufficiency. The final expression of the the seven genes that encode AMPK isoforms, we initially diabetic phenotype is strongly influenced by inheritance; chose PRKAA2, PRKAB1, and PRKAB2 because of their however, with the exception of rare monogenic forms of higher prior probability of association with type 2 diabetes, diabetes, common type 2 diabetes is thought to have a based on previous reports of genetic linkage, functional polygenic architecture (1). -

Systematic Screening for Potential Therapeutic Targets in Osteosarcoma Through a Kinome-Wide CRISPR-Cas9 Library
Cancer Biol Med 2020. doi: 10.20892/j.issn.2095-3941.2020.0162 ORIGINAL ARTICLE Systematic screening for potential therapeutic targets in osteosarcoma through a kinome-wide CRISPR-Cas9 library Yuanzhong Wu*, Liwen Zhou*, Zifeng Wang, Xin Wang, Ruhua Zhang, Lisi Zheng, Tiebang Kang Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China ABSTRACT Objective: Osteosarcoma is the most common primary malignant bone tumor. However, the survival of patients with osteosarcoma has remained unchanged during the past 30 years, owing to a lack of efficient therapeutic targets. Methods: We constructed a kinome-targeting CRISPR-Cas9 library containing 507 kinases and 100 nontargeting controls and screened the potential kinase targets in osteosarcoma. The CRISPR screening sequencing data were analyzed with the Model-based Analysis of Genome-wide CRISPR/Cas9 Knockout (MAGeCK) Python package. The functional data were applied in the 143B cell line through lenti-CRISPR-mediated gene knockout. The clinical significance of kinases in the survival of patients with osteosarcoma was analyzed in the R2: Genomics Analysis and Visualization Platform. Results: We identified 53 potential kinase targets in osteosarcoma. Among these targets, we analyzed 3 kinases, TRRAP, PKMYT1, and TP53RK, to validate their oncogenic functions in osteosarcoma. PKMYT1 and TP53RK showed higher expression in osteosarcoma than in normal bone tissue, whereas TRRAP showed no significant difference. High expression of all 3 kinases was associated with relatively poor prognosis in patients with osteosarcoma. Conclusions: Our results not only offer potential therapeutic kinase targets in osteosarcoma but also provide a paradigm for functional genetic screening by using a CRISPR-Cas9 library, including target design, library construction, screening workflow, data analysis, and functional validation. -

MAP2K3 (Human) Recombinant Protein (Q01)
MAP2K3 (Human) Recombinant phosphorylates and thus activates MAPK14/p38-MAPK. Protein (Q01) This kinase can be activated by insulin, and is necessary for the expression of glucose transporter. Expression of Catalog Number: H00005606-Q01 RAS oncogene is found to result in the accumulation of the active form of this kinase, which thus leads to the Regulation Status: For research use only (RUO) constitutive activation of MAPK14, and confers oncogenic transformation of primary cells. The inhibition Product Description: Human MAP2K3 partial ORF ( of this kinase is involved in the pathogenesis of Yersina AAH32478, 1 a.a. - 100 a.a.) recombinant protein with pseudotuberculosis. Multiple alternatively spliced GST-tag at N-terminal. transcript variants that encode distinct isoforms have been reported for this gene. [provided by RefSeq] Sequence: MESPASSQPASMPQSKGKSKRKKDLRISCMSKPPAP NPTPPRNLDSRTFITIGDRNFEVEADDLVTISELGRGAY GVVEKVRHAQSGTIMAVKRIRATVN Host: Wheat Germ (in vitro) Theoretical MW (kDa): 36.63 Applications: AP, Array, ELISA, WB-Re (See our web site product page for detailed applications information) Protocols: See our web site at http://www.abnova.com/support/protocols.asp or product page for detailed protocols Preparation Method: in vitro wheat germ expression system Purification: Glutathione Sepharose 4 Fast Flow Storage Buffer: 50 mM Tris-HCI, 10 mM reduced Glutathione, pH=8.0 in the elution buffer. Storage Instruction: Store at -80°C. Aliquot to avoid repeated freezing and thawing. Entrez GeneID: 5606 Gene Symbol: MAP2K3 Gene Alias: MAPKK3, MEK3, MKK3, PRKMK3 Gene Summary: The protein encoded by this gene is a dual specificity protein kinase that belongs to the MAP kinase kinase family. This kinase is activated by mitogenic and environmental stress, and participates in the MAP kinase-mediated signaling cascade. -

Human MAPK10 Peptide (DAG-P1762) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Human MAPK10 peptide (DAG-P1762) This product is for research use only and is not intended for diagnostic use. PRODUCT INFORMATION Antigen Description The protein encoded by this gene is a member of the MAP kinase family. MAP kinases act as an integration point for multiple biochemical signals, and are involved in a wide variety of cellular processes such as proliferation, differentiation, transcription regulation and development. This protein is a neuronal-specific form of c-Jun N-terminal kinases (JNKs). Through its phosphorylation and nuclear localization, this kinase plays regulatory roles in the signaling pathways during neuronal apoptosis. Beta-arrestin 2, a receptor-regulated MAP kinase scaffold protein, is found to interact with, and stimulate the phosphorylation of this kinase by MAP kinase kinase 4 (MKK4). Cyclin-dependent kianse 5 can phosphorylate, and inhibit the activity of this kinase, which may be important in preventing neuronal apoptosis. Four alternatively spliced transcript variants encoding distinct isoforms have been reported. [provided by RefSeq, Jul 2008] Specificity Specific to a subset of neurons in the nervous system. Present in the hippocampus and areas, cerebellum, striatum, brain stem, and weakly in the spinal cord. Very weak expression in testis and kidney. Nature Synthetic Expression System N/A Purity 70 - 90% by HPLC. Conjugate Unconjugated Sequence Similarities Belongs to the protein kinase superfamily. CMGC Ser/Thr protein kinase family. MAP kinase subfamily.Contains 1 protein kinase domain. Cellular Localization Cytoplasm. Procedure None Format Liquid Preservative None Storage Shipped at 4°C. Upon delivery aliquot and store at -20°C or -80°C. Avoid repeated freeze / thaw cycles. -

Mtor Coordinates Transcriptional Programs and Mitochondrial Metabolism of Activated Treg Subsets to Protect Tissue Homeostasis
ARTICLE DOI: 10.1038/s41467-018-04392-5 OPEN mTOR coordinates transcriptional programs and mitochondrial metabolism of activated Treg subsets to protect tissue homeostasis Nicole M. Chapman1, Hu Zeng 1, Thanh-Long M. Nguyen1, Yanyan Wang1, Peter Vogel 2, Yogesh Dhungana1, Xiaojing Liu3, Geoffrey Neale 4, Jason W. Locasale 3 & Hongbo Chi1 1234567890():,; Regulatory T (Treg) cells derived from the thymus (tTreg) and periphery (pTreg) have central and distinct functions in immunosuppression, but mechanisms for the generation and acti- vation of Treg subsets in vivo are unclear. Here, we show that mechanistic target of rapamycin (mTOR) unexpectedly supports the homeostasis and functional activation of tTreg and pTreg cells. mTOR signaling is crucial for programming activated Treg-cell function to protect immune tolerance and tissue homeostasis. Treg-specific deletion of mTOR drives sponta- neous effector T-cell activation and inflammation in barrier tissues and is associated with reduction in both thymic-derived effector Treg (eTreg) and pTreg cells. Mechanistically, mTOR functions downstream of antigenic signals to drive IRF4 expression and mitochondrial metabolism, and accordingly, deletion of mitochondrial transcription factor A (Tfam) severely impairs Treg-cell suppressive function and eTreg-cell generation. Collectively, our results show that mTOR coordinates transcriptional and metabolic programs in activated Treg subsets to mediate tissue homeostasis. 1 Department of Immunology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS 351, Memphis, TN 38105, USA. 2 Department of Pathology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS 250, Memphis, TN 38105, USA. 3 Department of Pharmacology & Cancer Biology, Duke University School of Medicine, Levine Science Research Center C266, Box 3813, Durham, NC 27710, USA. -

Genetics of Familial Non-Medullary Thyroid Carcinoma (FNMTC)
cancers Review Genetics of Familial Non-Medullary Thyroid Carcinoma (FNMTC) Chiara Diquigiovanni * and Elena Bonora Unit of Medical Genetics, Department of Medical and Surgical Sciences, University of Bologna, 40138 Bologna, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-051-208-8418 Simple Summary: Non-medullary thyroid carcinoma (NMTC) originates from thyroid follicular epithelial cells and is considered familial when occurs in two or more first-degree relatives of the patient, in the absence of predisposing environmental factors. Familial NMTC (FNMTC) cases show a high genetic heterogeneity, thus impairing the identification of pivotal molecular changes. In the past years, linkage-based approaches identified several susceptibility loci and variants associated with NMTC risk, however only few genes have been identified. The advent of next-generation sequencing technologies has improved the discovery of new predisposing genes. In this review we report the most significant genes where variants predispose to FNMTC, with the perspective that the integration of these new molecular findings in the clinical data of patients might allow an early detection and tailored therapy of the disease, optimizing patient management. Abstract: Non-medullary thyroid carcinoma (NMTC) is the most frequent endocrine tumor and originates from the follicular epithelial cells of the thyroid. Familial NMTC (FNMTC) has been defined in pedigrees where two or more first-degree relatives of the patient present the disease in absence of other predisposing environmental factors. Compared to sporadic cases, FNMTCs are often multifocal, recurring more frequently and showing an early age at onset with a worse outcome. FNMTC cases Citation: Diquigiovanni, C.; Bonora, E. -

Identifying Novel Actionable Targets in Colon Cancer
biomedicines Review Identifying Novel Actionable Targets in Colon Cancer Maria Grazia Cerrito and Emanuela Grassilli * Department of Medicine and Surgery, University of Milano-Bicocca, Via Cadore 48, 20900 Monza, Italy; [email protected] * Correspondence: [email protected] Abstract: Colorectal cancer is the fourth cause of death from cancer worldwide, mainly due to the high incidence of drug-resistance toward classic chemotherapeutic and newly targeted drugs. In the last decade or so, the development of novel high-throughput approaches, both genome-wide and chemical, allowed the identification of novel actionable targets and the development of the relative specific inhibitors to be used either to re-sensitize drug-resistant tumors (in combination with chemotherapy) or to be synthetic lethal for tumors with specific oncogenic mutations. Finally, high- throughput screening using FDA-approved libraries of “known” drugs uncovered new therapeutic applications of drugs (used alone or in combination) that have been in the clinic for decades for treating non-cancerous diseases (re-positioning or re-purposing approach). Thus, several novel actionable targets have been identified and some of them are already being tested in clinical trials, indicating that high-throughput approaches, especially those involving drug re-positioning, may lead in a near future to significant improvement of the therapy for colon cancer patients, especially in the context of a personalized approach, i.e., in defined subgroups of patients whose tumors carry certain mutations. Keywords: colon cancer; drug resistance; target therapy; high-throughput screen; si/sh-RNA screen; CRISPR/Cas9 knockout screen; drug re-purposing; drug re-positioning Citation: Cerrito, M.G.; Grassilli, E. -

Support Info
Electronic Supplementary Material (ESI) for RSC Advances. This journal is © The Royal Society of Chemistry 2014 Supporting Information Design and synthesis of pyrrole–5-(2,6-dichlorobenzyl)sulfonylindolin-2-ones with C- 3’ side chains as potent Met kinase inhibitors Chia-Wei Liu,a Chun-Liang Lai,a Yu-Hsiang Lin,a Li-Wei Teng,a Sheng-chuan Yang,a Win-Yin Wei,a Shu Fu Lin,a Ju-Ying Yang,a Hung-Jyun Huang,a Ru-Wen Wang,a Chao-Cheng Chiang,a Mei-Hui Lee,a Yu- Chuan Wang,b Shih-Hsien Chuang,a Jia-Ming Chang,a Ying-Shuan E. Lee,a and Jiann-Jyh Huang*a,b aDevelopment Center for Biotechnology, No. 101, Lane 169, Kangning St., Xizhi District, New Taipei City 22180, Taiwan bDepartment of Applied Chemistry, National Chiayi University, No. 300, Syuefu Rd., Chiayi City 60004, Taiwan *Corresponding Author. Tel.: +886 5 271 7959; Fax: +886 5 271 7901. E-mail address: [email protected] (J.-J. Huang) Table of Contents: Page Supporting Figure. Ligplot diagrams of the ATP binding site of Met S2 complexed with compounds 2 and 20. Supporting Table. Kinase profiling data of compound 20. S3 References S10 - S1 - Supporting Figure. Ligplot diagrams1 of the ATP binding site of Met complexed with compounds 2 and 20: (A) Met with 2, and (B) Met with 20. - S2 - Supporting Table. Kinase profiling data of 20. Ambit KinomeScan Kinase Profiling (1.0 μM test concentration): Percentage of Percentage of Ambit Gene Symbol control (%) Ambit Gene Symbol control (%) 20 20 AAK1 68 ARK5 27 ABL1(E255K)-phosphorylated 85 ASK1 100 ABL1(F317I)-nonphosphorylated 78 ASK2 67 -

Profiling Data
Compound Name DiscoveRx Gene Symbol Entrez Gene Percent Compound Symbol Control Concentration (nM) JNK-IN-8 AAK1 AAK1 69 1000 JNK-IN-8 ABL1(E255K)-phosphorylated ABL1 100 1000 JNK-IN-8 ABL1(F317I)-nonphosphorylated ABL1 87 1000 JNK-IN-8 ABL1(F317I)-phosphorylated ABL1 100 1000 JNK-IN-8 ABL1(F317L)-nonphosphorylated ABL1 65 1000 JNK-IN-8 ABL1(F317L)-phosphorylated ABL1 61 1000 JNK-IN-8 ABL1(H396P)-nonphosphorylated ABL1 42 1000 JNK-IN-8 ABL1(H396P)-phosphorylated ABL1 60 1000 JNK-IN-8 ABL1(M351T)-phosphorylated ABL1 81 1000 JNK-IN-8 ABL1(Q252H)-nonphosphorylated ABL1 100 1000 JNK-IN-8 ABL1(Q252H)-phosphorylated ABL1 56 1000 JNK-IN-8 ABL1(T315I)-nonphosphorylated ABL1 100 1000 JNK-IN-8 ABL1(T315I)-phosphorylated ABL1 92 1000 JNK-IN-8 ABL1(Y253F)-phosphorylated ABL1 71 1000 JNK-IN-8 ABL1-nonphosphorylated ABL1 97 1000 JNK-IN-8 ABL1-phosphorylated ABL1 100 1000 JNK-IN-8 ABL2 ABL2 97 1000 JNK-IN-8 ACVR1 ACVR1 100 1000 JNK-IN-8 ACVR1B ACVR1B 88 1000 JNK-IN-8 ACVR2A ACVR2A 100 1000 JNK-IN-8 ACVR2B ACVR2B 100 1000 JNK-IN-8 ACVRL1 ACVRL1 96 1000 JNK-IN-8 ADCK3 CABC1 100 1000 JNK-IN-8 ADCK4 ADCK4 93 1000 JNK-IN-8 AKT1 AKT1 100 1000 JNK-IN-8 AKT2 AKT2 100 1000 JNK-IN-8 AKT3 AKT3 100 1000 JNK-IN-8 ALK ALK 85 1000 JNK-IN-8 AMPK-alpha1 PRKAA1 100 1000 JNK-IN-8 AMPK-alpha2 PRKAA2 84 1000 JNK-IN-8 ANKK1 ANKK1 75 1000 JNK-IN-8 ARK5 NUAK1 100 1000 JNK-IN-8 ASK1 MAP3K5 100 1000 JNK-IN-8 ASK2 MAP3K6 93 1000 JNK-IN-8 AURKA AURKA 100 1000 JNK-IN-8 AURKA AURKA 84 1000 JNK-IN-8 AURKB AURKB 83 1000 JNK-IN-8 AURKB AURKB 96 1000 JNK-IN-8 AURKC AURKC 95 1000 JNK-IN-8 -

Application of a MYC Degradation
SCIENCE SIGNALING | RESEARCH ARTICLE CANCER Copyright © 2019 The Authors, some rights reserved; Application of a MYC degradation screen identifies exclusive licensee American Association sensitivity to CDK9 inhibitors in KRAS-mutant for the Advancement of Science. No claim pancreatic cancer to original U.S. Devon R. Blake1, Angelina V. Vaseva2, Richard G. Hodge2, McKenzie P. Kline3, Thomas S. K. Gilbert1,4, Government Works Vikas Tyagi5, Daowei Huang5, Gabrielle C. Whiten5, Jacob E. Larson5, Xiaodong Wang2,5, Kenneth H. Pearce5, Laura E. Herring1,4, Lee M. Graves1,2,4, Stephen V. Frye2,5, Michael J. Emanuele1,2, Adrienne D. Cox1,2,6, Channing J. Der1,2* Stabilization of the MYC oncoprotein by KRAS signaling critically promotes the growth of pancreatic ductal adeno- carcinoma (PDAC). Thus, understanding how MYC protein stability is regulated may lead to effective therapies. Here, we used a previously developed, flow cytometry–based assay that screened a library of >800 protein kinase inhibitors and identified compounds that promoted either the stability or degradation of MYC in a KRAS-mutant PDAC cell line. We validated compounds that stabilized or destabilized MYC and then focused on one compound, Downloaded from UNC10112785, that induced the substantial loss of MYC protein in both two-dimensional (2D) and 3D cell cultures. We determined that this compound is a potent CDK9 inhibitor with a previously uncharacterized scaffold, caused MYC loss through both transcriptional and posttranslational mechanisms, and suppresses PDAC anchorage- dependent and anchorage-independent growth. We discovered that CDK9 enhanced MYC protein stability 62 through a previously unknown, KRAS-independent mechanism involving direct phosphorylation of MYC at Ser .