Guerrero Et Al 2006
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

(Lonicera L.) Genties Atstovų Genetinės Įvairovės Ir Filogenetiniai Tyrimai Dnr Ţymenų Metodais
VILNIAUS UNIVERSITETAS Donatas Naugţemys SAUSMEDŢIO (LONICERA L.) GENTIES ATSTOVŲ GENETINĖS ĮVAIROVĖS IR FILOGENETINIAI TYRIMAI DNR ŢYMENŲ METODAIS Daktaro disertacija Biomedicinos mokslai, biologija (01 B) Vilnius, 2011 Disertacija rengta 2006 – 2010 metais Vilniaus universitete. Mokslinis vadovas: prof. dr. Donatas Ţvingila (Vilniaus universitetas, biomedicinos mokslai, biologija – 01 B) Konsultantas: dr. Silva Ţilinskaitė (Vilniaus universitetas, biomedicinos mokslai, biologija – 01 B) 2 TURINYS SANTRUMPOS ..................................................................................................... 5 ĮVADAS ................................................................................................................. 7 I. LITERATŪROS APŢVALGA ......................................................................... 13 1. Sausmedţio genties apţvalga ....................................................................... 13 1.1. Lonicera L. genties sistematikos istorija ir problemos .......................... 15 1.2. Lonicera L. genties kilmė ...................................................................... 21 2. Molekuliniai ţymenys ir augalų filogenetiniai tyrimai ................................ 24 2.1. RAPD metodo taikymas augalų sistematikoje ...................................... 26 2.2. Chloroplastų DNR nekoduojančių specifinių regionų tyrimas sekoskaitos metodu .............................................................................................. 31 2.3. Lonicera L. genties filogenetikos molekuliniai tyrimai -

Euphydryas Aurinia (Lepidoptera: Nymphalinae: Melitaeini) Select the Greenest Leaves of Lonicera Implexa (Caprifoliaceae) for Oviposition
Eur. J. Entomol. 103: 569–574, 2006 ISSN 1210-5759 Females of the specialist butterfly Euphydryas aurinia (Lepidoptera: Nymphalinae: Melitaeini) select the greenest leaves of Lonicera implexa (Caprifoliaceae) for oviposition CONSTANTÍ STEFANESCU1, JOSEP PEÑUELAS2, JORDI SARDANS2 and IOLANDA FILELLA2 1Butterfly Monitoring Scheme, Museu de Granollers-Ciències Naturals, Francesc Macià 51, E-08400 Granollers, Spain; e-mail: [email protected] 2Ecophysiology Unit CSIC-CEAB-CREAF, CREAF (Center for Ecological Research and Forestry Applications), Edifici C, Universitat Autònoma de Barcelona, 08193 Bellaterra, Barcelona, Spain; e-mails: [email protected], [email protected], [email protected] Key words. Lepidoptera, Nymphalinae, Euphydryas aurinia, Lonicera implexa, insect-plant interaction, oviposition cues, plant size, foliar chlorophyll concentration, spectral reflectance, Mediterranean area Abstract. In Mediterranean habitats, the specialist butterfly Euphydryas aurinia oviposits on Lonicera implexa. Previous work has shown that ovipositing females select and lay a higher number of egg clusters on certain plants. In this paper the results of a field study aimed at assessing whether females use plant size and/or plant or leaf greenness (i.e., chlorophyll concentrations) as cues for oviposition are described. Size of plants did not appear to be an important factor in determining host plant selection, probably because even small plants provide enough resources for the young larvae to reach the diapausing stage and because last instar larvae, the most likely to face resource depletion, can move great distances in search of food. Measurements of both spectral reflectance and chlorophyll concentration of plants failed to reveal differences between host and non-host plants. On the other hand, reflectance and chlorophyll concentration of leaves were found to be important in oviposition choice as egg clusters were generally located on the greenest leaves with the highest chlorophyll contents. -

E. Biondi, L. Gubellini, M. Pinzi & S. Casavecchia the Vascular Flora Of
Fl. Medit. 22: 67-167 doi: 10.7320/FlMedit22.067 Version of Record published online on 28 December 2012 E. Biondi, L. Gubellini, M. Pinzi & S. Casavecchia The vascular flora of Conero Regional Nature Park (Marche, Central Italy) To Aldo J. B. Brilli-Cattarini, eminent botanist and expert resear- cher on the Marche region flora and par- ticularly on the Conero flora. He was the founder of the “Centro Ricerche Floristiche Marche” of Pesaro and Urbino Province. He has been a master for all of us even in the preparation of this work, with great gene rosity, he offered us his unique experience with enthusiasm. Abstract Biondi E., Gubellini L., Pinzi M. & Casavecchia, S.: The vascular flora of Conero Regional Nature Park (Marche, Central Italy). — Fl. Medit. 22: 67-167. 2012. — ISSN: 1120-4052 print- ed, 2240-4538 online. It is presented the vascular flora of Conero Regional Park. The paper starts with a brief presen- tation of the park territory (geography, geomorphology and bioclimate) that arises in the cen- tral Adriatic side of the Italian peninsula. The vegetation is briefly described thanks to the detailed phytosociological analysis carried out in the territory and to detailed maps on different scales. The description of vegetation allows indicating, for most of the species listed, their par- ticipation in the plant communities present in the area. The floristic list comprises 1169 entities, of specific and sub specific levels, that belong to 101 families and 507 genera. It also includes 64 species currently disappeared or not recently found in the study area, indicated by NP acronym (not present). -

Candidates for the Biological Control of Teasel, Dipsacus Spp
Candidates for the biological control of teasel, Dipsacus spp. René Sforza1 Summary Dipsacus fullonum L., wild teasel, and D. laciniatus L., cut-leaf teasel (Dipsacaceae), native to Eurasia, were introduced into North America in the 1700s. Primarily cultivated for its seedheads, D. fullonum escaped from cultivation and colonized waterways, waste ground, fallow fields and pastures, outcom- peting native plants. This study reports on foreign exploration for biological control agents against teasel in its native range. Countries from France to Russia were surveyed, with a particular emphasis on insects feeding either on rosettes or seedheads. Two potential candidates were collected, namely the checkerspot butterfly, Euphydryas aurinia (Lepidoptera: Nymphalidae), and the leaf beetle, Galeruca pomonae (Coleoptera: Chrysomelidae). This is the first report of these two insect species feeding on teasel. Both were collected in the same locations in northern Turkey, and may feed concurrently on the same plants. Galeruca pomonae was also collected from south-eastern Russia. Preliminary host-choice and no-choice test experiments showed that G. pomonae can complete its entire development on teasel but does not feed on carrot, radish, cabbage, or lettuce. Euphydryas aurinia populations from Turkey were parasitized by a tachinid fly, Erycia furibunda. Ecological considerations and host specificity are discussed for potential biological control programs. Keywords: Biocontrol, Chrysomelidae, Dipsacaceae, Endothenia, Euphydryas, Galeruca, invasion, Nymphalidae, teasel, Tortricidae, weeds. Introduction 1975b). This plant belongs to the Dipsacaceae family (300 species worldwide) divided into three tribes, Teasel is an invasive species in North America. which comprise only 12 North American species Research on herbivores of Dipsacus fullonum and among the genera Dipsacus, Knautia, Succisella, closely related species has been conducted since 2000. -

Evolutionary Transitions of Style Polymorphisms in Lithodora (Boraginaceae) V
ARTICLE IN PRESS Perspectives in Plant Ecology, Evolution and Systematics Perspectives in Plant Ecology, Evolution and Systematics 11 (2009) 111–125 www.elsevier.de/ppees Evolutionary transitions of style polymorphisms in Lithodora (Boraginaceae) V. Ferreroa,Ã, J. Arroyob, P. Vargasc, J.D. Thompsond, L. Navarroa aDepartamento de Biologı´a Vegetal y Ciencias del Suelo, Facultad de Biologı´a, Universidad de Vigo, As Lagoas-Marcosende 36200 Vigo, Spain bDepartamento de Biologı´a Vegetal y Ecologı´a, Universidad de Sevilla, Apartado 1095, E-41080 Sevilla, Spain cReal Jardı´n Bota´nico de Madrid, CSIC, Plaza Murillo 2, 28014 Madrid, Spain dUMR 5175 Centre d’Ecologie Fonctionnelle et Evolutive, CNRS, 1919 Route de Mende, F-34293 Montpellier Cedex 5, France Received 22 September 2008; received in revised form 10 January 2009; accepted 19 January 2009 Abstract Floral polymorphisms provide suitable model systems to test hypotheses concerning the evolution of outbreeding in plants. Although heterostyly has evolved in more than 28 angiosperm families, the evolutionary pathways involving related floral conditions have not yet been fully resolved. In this study, the reconstruction of ancestral states of style polymorphism, with both parsimony and maximum likelihood methods, was carried out for Boraginaceae species in the tribe Lithospermeae, particularly in the genus Lithodora sensu lato, where species present a wide variety of stylar conditions. Detailed floral morphometric analysis confirm different types of style polymorphism within Lithodora. They also reveal a novel style polymorphism (relaxed style dimorphism) in which anther height is variable within a flower (each anther being at a different height), which contrasts to regular distyly (constant anther height within flowers). -
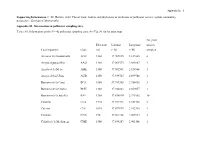
Supporting Information. C. M. Herrera. 2020. Flower Traits, Habitat, and Phylogeny As Predictors of Pollinator Service: a Plant Community Perspective
Appendix S1 – 1 Supporting Information. C. M. Herrera. 2020. Flower traits, habitat, and phylogeny as predictors of pollinator service: a plant community perspective. Ecological Monographs. Appendix S1. Information on pollinator sampling sites. TABLE S1. Information on the N = 42 pollinator sampling sites. See Fig. S1 for location map. No. plant Elevation Latitude Longitude species Local toponym Code (m) (º N) (º W) sampled Arenales del Guadalentín AGU 1360 37.909835 2.837405 4 Arroyo Aguaderillos AAG 1180 37.961573 2.883887 1 Arroyo de la Mesa AME 1100 37.902591 2.928966 1 Arroyo de los Ubios AUB 1250 37.939725 2.899766 1 Barranco de la Canal BCA 1580 37.789385 2.956438 3 Barranco de la Charca BCH 1360 37.942843 2.859057 1 Barranco de la Juanfría BJU 1380 37.836814 2.975302 10 Calarilla CLA 1710 37.944316 2.851708 2 Calerón CLE 1075 37.897973 2.942710 1 Cantalar CAN 770 37.964158 2.907974 3 Cañada de la Medianega CME 1580 37.894287 2.903146 1 Appendix S1 – 2 Cañada Pajarera CPA 1640 37.927456 2.805615 1 Collado del Calvario CCA 1420 37.950202 2.885484 1 Collado Zamora CZA 1420 37.839329 2.999615 2 Coto del Valle CVA 850 37.930542 2.928517 7 Cuesta del Bazar CBA 1330 37.906523 2.907470 1 Cuevas Bermejas CBE 1160 37.966959 2.851117 5 Fuente Bermejo FBE 1550 37.932268 2.840672 14 Fuente la Reina FRE 1460 37.941698 2.833632 2 Guadalentín GUA 1310 37.900404 2.836944 8 Hoyos de Muñoz HMU 1110 37.984831 2.876597 3 La Cabrilla LCA 1600 37.932364 2.780637 5 La Fresnedilla LFR 1080 37.980387 2.855875 2 La Mesa LME 1610 37.889823 2.913788 2 Las -

Reproductive Characteristics As Drivers of Alien Plant Naturalization and Invasion
Reproductive characteristics as drivers of alien plant naturalization and invasion Dissertation submitted for the degree of Doctor of Natural Sciences presented by Mialy Harindra Razanajatovo at the Faculty of Sciences Department of Biology Date of the oral examination: 12 February 2016 First referee: Prof. Dr. Mark van Kleunen Second referee: Prof. Dr. Markus Fischer Konstanzer Online-Publikations-System (KOPS) URL: http://nbn-resolving.de/urn:nbn:de:bsz:352-0-324483 Summary Due to human activity and global movements, many plant species have been introduced to non-native regions where they experience novel abiotic and biotic conditions. Some of these alien species manage to establish reproducing naturalized populations, and some naturalized alien species subsequently become invasive. Invasion by alien plant species can negatively affect native communities and ecosystems, but what gives the alien species an advantage under novel conditions is still not clear. Therefore, identifying the drivers of invasions has become a major goal in invasion ecology. Reproduction is crucial in plant invasions, because propagule supply is required for founding new populations, population maintenance and spread in non-native regions. Baker’s Law, referring to the superior advantage of species capable of uniparental reproduction in establishing after long distance dispersal, has received major interest in explaining plant invasions. However, previous findings regarding Baker’s Law are contradicting. Moreover, there has been an increasing interest in understanding the integration of alien plant species into native plant-pollinator networks but few studies have looked at the pollination ecology of successful (naturalized and invasive) and unsuccessful (non-naturalized and non-invasive) alien plant species. -

A Phylogenetic Analysis of Dipsacaceae Based on Four DNA Regions
Plant Syst Evol (2009) 279:69–86 DOI 10.1007/s00606-009-0147-y ORIGINAL ARTICLE A phylogenetic analysis of Dipsacaceae based on four DNA regions M. Avino Æ G. Tortoriello Æ P. Caputo Received: 30 July 2008 / Accepted: 4 January 2009 / Published online: 31 March 2009 Ó Springer-Verlag 2009 Abstract Authors studied the phylogeny of Dipsacaceae molecular grounds. Lack of evident synapomorphies for using maximum parsimony and Bayesian analyses on various clades is interpreted as a possible consequence of fast sequence data from chloroplast (trnL intron, trnL–trnF adaptative radiation. intergenic spacer, psbB–psbH gene complex) and nuclear genomes (ITS1 and ITS2). Both data partitions as well as Keywords Dipsacaceae Á Internal transcribed their combination show that Dipsacaceae is a monophyletic spacers (ITS) Á Phylogeny Á psbB–psbH Á trnL intron Á group. Topology in tribe Scabioseae is similar to those of trnL–trnF intergenic spacer other recent studies, except for the position of Pycnocomon, which is nested in Lomelosia. Pycnocomon, the pollen and epicalyx morphologies of which closely resemble those of Introduction Lomelosia, is interpreted as a psammophilous morphotype of Lomelosia, and its nomenclature has been revised accord- Dipsacaceae Juss. (Dipsacales Lindl.), the teasel family, ingly. Exclusion of Pseudoscabiosa, Pterocephalidium, includes 250–350 species (Ehrendorfer 1965; Verla´que Pterocephalodes (and probably Bassecoia), Succisa, Succi- 1977a) of annual to perennial herbs and shrubs; native sella from Scabioseae is confirmed. Pterocephalodes mostly to Mediterranean and temperate climates, they are hookeri is the sister group to the rest of the family. Its found in north temperate Eurasia and in tropical to southern remoteness from Pterocephalus has been confirmed on Africa. -

1 He Floras of Gibraltar
Comunicaciones 1 HE FLORAS OF GIBRALTAR John Cortés /Doctor en Biología por la Universidad de Oxford Abstract The spectacular appearance of the Rock of Gibraltar, as well as the consequences of its military and political history have meant that it has attracted more attention in manyfields than similarsized mountains in the region and than most in Iberia. This extends to theflora. Apartffom a good number of species listsproduced by collectorsfiom the 18th Century, there exista number of more or less complete Floras of the Rock. Five of these have beenproduced,viz. Kelaart (1846), Debeaux & Dautez (1888), Frere (191O), and Wolley Dod (1914) and then, after a break of eigh~years, Linares (1990 & 1993). This paper discusses the different approaches and relative merits of thesefloras as historical and botanical documents, and, taking Wolley Dod contribution as a starting poinr, compares it with the present flora of Gibraltar reaching conclusions on the changes and making predictions for the future. Resumen Quizás por su aspecto conocido y impresionante, o tal vez por los accidentes de su historia militar y política, el Peñón de Gibraltar ha recibido más atención botánica desde el siglo pasado que ninguna otra sierra de la zona y que de muchas en la Península Ibérica. Aparte de numerosas referencias botánicas de coleccionistas durante más de un siglo, se han publicado cinco "listas completas" de laflora de Gibraltar, lasfloras de Kelaart (1846), Debeaux Q Dautez (1888), Frere (191O), Wolley Dod (1914), y, tras más de medio siglo, Linares (1990 y 1993). Comunicaciones Esta contribución compara las obras de estos botánicos y, partiendo de2 trabajo histórico de Wolley Dod, compara laflora que encontró él en Gibraltar en 1914 con la del presente, llegando a conclusiones sobre las cambios que han habido en ocho décadas y las lecciones para el fiituro. -

CXXXVIII. BORAGINACEAE [Nom
CXXXVIII. BORAGINACEAE [nom. cons.]* Hierbas o subarbustos –en especies extraibéricas también árboles– con indu- mento setoso-híspido, de setas unicelulares y blancas de base pustulado-tuber- culada, muy rara vez glabras o seríceas, a veces acompañadas de pelos plurice- lulares glandulíferos y eglandulosos. Tallo de sección circular, folioso. Hojas al- ternas, rara vez opuestas, enteras, rara vez sinuado-dentadas, con nerviación pinnada, sin estípulas, las inferiores normalmente pecioladas y frecuentemente formando una roseta ± marcada –en la base de plantas anuales o bienales–, las caulinares pecioladas o sésiles, a veces decurrentes por el tallo. Inflorescencia cimosa, con cimas escorpioides, frecuentemente geminadas, generalmente den- sas en la floración y laxas o densas en la fructificación. Flores hermafroditas, rara vez femeninas, pentámeras –en especies extraibéricas tetrámeras o 10-12- meras–, actinomorfas o zigomorfas, diclamídeas, hipóginas, pediceladas o sési- les, bracteadas o ebracteadas. Cáliz gamosépalo, con 5 sépalos –lóbulos– a ve- ces separados casi hasta la base, normalmente acrescente en la fructificación, muy rara vez dialisépalo con los sépalos en disposición helicoidal. Corola ga- mopétala, con 5 pétalos, rotácea, estrellada, campanulada, urceolada, en tubo, infundibuliforme o hipocrateriforme, generalmente con un tubo y un limbo bien diferenciados; tubo normalmente cerrado por 5 escamas o invaginaciones opuestas a los lóbulos de la corola o con un anillo de pelos o de papilas en la parte superior (garganta), a veces con un anillo interno de escamas en la base relacionadas con el acceso al néctar de la flor –escamas nectaríferas–; limbo con 5 lóbulos ± marcados en las flores actinomorfas, a veces muy pequeños y reflejos en las corolas campanuladas o urceoladas, o no muy bien definidos en las muy zigomorfas. -

To Download the Food Plant Database in PDF Format
HOST PLANT PLANT FAMILY FEEDING NICHE HERBIVORE SUBFAMILY REFERENCE GEOREGION LOCATION Abelia spathulata Siebold & Zucc. Caprifoliaceae Acleris askoldana (Christoph) Tortricinae Yasuda 1972 Asia Abelmoschus esculentus (L.) Malvaceae Crocidosema plebejana Zeller Olethreutinae Heinrich 1921; Diakonoff 1982; Nasu & Yasuda 1993 Asia Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Crocidosema plebejana Zeller Olethreutinae Heinrich 1921; Diakonoff 1982 North America Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Platynota nigrocervina Walsingham Tortricinae MacKay 1962a North America Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Platynota rostrana (Walker) Tortricinae Heinrich 1921 North America Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Archips micaceana (Walker) Tortricinae Pholboon 1965; Kuroko & Lewvanich 1993 Asia Moench Abelmoschus esculentus (L.) Malvaceae Archips philippa (Meyrick) Tortricinae BMNH collection Asia India Moench Abelmoschus esculentus (L.) Malvaceae Homona tabescens (Meyrick) Tortricinae Yunus & Ho 1980 Asia Malaysia Moench Abelmoschus esculentus Moench Malvaceae Crocidosema plebejana Zeller Olethreutinae Fletcher 1932 Asia India (as Hibiscus) Abelmoschus esculentus Moench Thaumatotibia leucotreta (Meyrick) (as Malvaceae Olethreutinae Whittle 1984 Africa (as Hibiscus) Cryptophlebia) Abies alba Mill. Pinaceae Acleris variana (Fernald) Tortricinae Meyrick MS 1938 North America Abies alba Mill. Pinaceae Archips oporana (Linnaeus) Tortricinae Bradley et al. 1973 Europe Abies -
Effects of Habitat Quality, Diversity and Fragmentation Inge Van Halder
Conservation of butterfly communities in mosaic forest landscapes : effects of habitat quality, diversity and fragmentation Inge van Halder To cite this version: Inge van Halder. Conservation of butterfly communities in mosaic forest landscapes : effects of habitat quality, diversity and fragmentation. Ecosystems. Université de Bordeaux, 2017. English. NNT : 2017BORD0001. tel-01477782 HAL Id: tel-01477782 https://tel.archives-ouvertes.fr/tel-01477782 Submitted on 27 Feb 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE PRÉSENTÉE POUR OBTENIR LE GRADE DE DOCTEUR DE L’UNIVERSITÉ DE BORDEAUX ÉCOLE DOCTORALE SCIENCES ET ENVIRONNEMENTS SPECIALITÉ : Ecologie évolutive, fonctionnelle et des communautés Par Inge VAN HALDER Conservation des communautés de papillons de jour dans les paysages forestiers hétérogènes : effets de la qualité, de la diversité et de la fragmentation des habitats Sous la direction de : Hervé JACTEL co-directeur : Luc BARBARO Soutenue le 6 janvier 2017 Membres du jury : Mme BUREL, Françoise Directeur de recherches, CNRS Rennes Rapporteur Mme PETIT, Sandrine Directeur de recherches, INRA Dijon Rapporteur M. DUFRÊNE, Marc Professeur, Université de Liège Examinateur Mme SIRAMI, Clélia Chargé de recherches, INRA Toulouse Examinateur M. JACTEL, Hervé Directeur de recherches, INRA Bordeaux Directeur M.