Evolutionary Transitions of Style Polymorphisms in Lithodora (Boraginaceae) V
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conceptual Design Documentation
Appendix A: Conceptual Design Documentation APPENDIX A Conceptual Design Documentation June 2019 A-1 APPENDIX A: CONCEPTUAL DESIGN DOCUMENTATION The environmental analyses in the NEPA and CEQA documents for the proposed improvements at Oceano County Airport (the Airport) are based on conceptual designs prepared to provide a realistic basis for assessing their environmental consequences. 1. Widen runway from 50 to 60 feet 2. Widen Taxiways A, A-1, A-2, A-3, and A-4 from 20 to 25 feet 3. Relocate segmented circle and wind cone 4. Installation of taxiway edge lighting 5. Installation of hold position signage 6. Installation of a new electrical vault and connections 7. Installation of a pollution control facility (wash rack) CIVIL ENGINEERING CALCULATIONS The purpose of this conceptual design effort is to identify the amount of impervious surface, grading (cut and fill) and drainage implications of the projects identified above. The conceptual design calculations detailed in the following figures indicate that Projects 1 and 2, widening the runways and taxiways would increase the total amount of impervious surface on the Airport by 32,016 square feet, or 0.73 acres; a 6.6 percent increase in the Airport’s impervious surface area. Drainage patterns would remain the same as both the runway and taxiways would continue to sheet flow from their centerlines to the edge of pavement and then into open, grassed areas. The existing drainage system is able to accommodate the modest increase in stormwater runoff that would occur, particularly as soil conditions on the Airport are conducive to infiltration. Figure A-1 shows the locations of the seven projects incorporated in the Proposed Action. -

Conserving Europe's Threatened Plants
Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation By Suzanne Sharrock and Meirion Jones May 2009 Recommended citation: Sharrock, S. and Jones, M., 2009. Conserving Europe’s threatened plants: Progress towards Target 8 of the Global Strategy for Plant Conservation Botanic Gardens Conservation International, Richmond, UK ISBN 978-1-905164-30-1 Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, Surrey, TW9 3BW, UK Design: John Morgan, [email protected] Acknowledgements The work of establishing a consolidated list of threatened Photo credits European plants was first initiated by Hugh Synge who developed the original database on which this report is based. All images are credited to BGCI with the exceptions of: We are most grateful to Hugh for providing this database to page 5, Nikos Krigas; page 8. Christophe Libert; page 10, BGCI and advising on further development of the list. The Pawel Kos; page 12 (upper), Nikos Krigas; page 14: James exacting task of inputting data from national Red Lists was Hitchmough; page 16 (lower), Jože Bavcon; page 17 (upper), carried out by Chris Cockel and without his dedicated work, the Nkos Krigas; page 20 (upper), Anca Sarbu; page 21, Nikos list would not have been completed. Thank you for your efforts Krigas; page 22 (upper) Simon Williams; page 22 (lower), RBG Chris. We are grateful to all the members of the European Kew; page 23 (upper), Jo Packet; page 23 (lower), Sandrine Botanic Gardens Consortium and other colleagues from Europe Godefroid; page 24 (upper) Jože Bavcon; page 24 (lower), Frank who provided essential advice, guidance and supplementary Scumacher; page 25 (upper) Michael Burkart; page 25, (lower) information on the species included in the database. -

Two New Genera in the Omphalodes Group (Cynoglosseae, Boraginaceae)
Nova Acta Científica Compostelana (Bioloxía),23 : 1-14 (2016) - ISSN 1130-9717 ARTÍCULO DE INVESTIGACIÓN Two new genera in the Omphalodes group (Cynoglosseae, Boraginaceae) Dous novos xéneros no grupo Omphalodes (Cynoglosseae, Boraginaceae) M. SERRANO1, R. CARBAJAL1, A. PEREIRA COUTINHO2, S. ORTIZ1 1 Department of Botany, Faculty of Pharmacy, University of Santiago de Compostela, 15782 Santiago de Compostela , Spain 2 CFE, Centre for Functional Ecology, Department of Life Sciences, University of Coimbra, 3000-456 Coimbra, Portugal *[email protected]; [email protected]; [email protected]; [email protected] *: Corresponding author (Recibido: 08/06/2015; Aceptado: 01/02/2016; Publicado on-line: 04/02/2016) Abstract Omphalodes (Boraginaceae, Cynoglosseae) molecular phylogenetic relationships are surveyed in the context of the tribe Cynoglosseae, being confirmed that genusOmphalodes is paraphyletic. Our work is focused both in the internal relationships among representatives of Omphalodes main subgroups (and including Omphalodes verna, the type species), and their relationships with other Cynoglosseae genera that have been related to the Omphalodes group. Our phylogenetic analysis of ITS and trnL-trnF molecular markers establish close relationships of the American Omphalodes with the genus Mimophytum, and also with Cynoglossum paniculatum and Myosotidium hortensia. The southwestern European annual Omphalodes species form a discrete group deserving taxonomic recognition. We describe two new genera to reduce the paraphyly in the genus Omphalodes, accommodating the European annual species in Iberodes and Cynoglossum paniculatum in Mapuchea. The pollen of the former taxon is described in detail for the first time. Keywords: Madrean-Tethyan, phylogeny, pollen, systematics, taxonomy Resumo Neste estudo analisamos as relacións filoxenéticas deOmphalodes (Boraginaceae, Cynoglosseae) no contexto da tribo Cynoglosseae, confirmándose como parafilético o xéneroOmphalodes . -

Flora Mediterranea 26
FLORA MEDITERRANEA 26 Published under the auspices of OPTIMA by the Herbarium Mediterraneum Panormitanum Palermo – 2016 FLORA MEDITERRANEA Edited on behalf of the International Foundation pro Herbario Mediterraneo by Francesco M. Raimondo, Werner Greuter & Gianniantonio Domina Editorial board G. Domina (Palermo), F. Garbari (Pisa), W. Greuter (Berlin), S. L. Jury (Reading), G. Kamari (Patras), P. Mazzola (Palermo), S. Pignatti (Roma), F. M. Raimondo (Palermo), C. Salmeri (Palermo), B. Valdés (Sevilla), G. Venturella (Palermo). Advisory Committee P. V. Arrigoni (Firenze) P. Küpfer (Neuchatel) H. M. Burdet (Genève) J. Mathez (Montpellier) A. Carapezza (Palermo) G. Moggi (Firenze) C. D. K. Cook (Zurich) E. Nardi (Firenze) R. Courtecuisse (Lille) P. L. Nimis (Trieste) V. Demoulin (Liège) D. Phitos (Patras) F. Ehrendorfer (Wien) L. Poldini (Trieste) M. Erben (Munchen) R. M. Ros Espín (Murcia) G. Giaccone (Catania) A. Strid (Copenhagen) V. H. Heywood (Reading) B. Zimmer (Berlin) Editorial Office Editorial assistance: A. M. Mannino Editorial secretariat: V. Spadaro & P. Campisi Layout & Tecnical editing: E. Di Gristina & F. La Sorte Design: V. Magro & L. C. Raimondo Redazione di "Flora Mediterranea" Herbarium Mediterraneum Panormitanum, Università di Palermo Via Lincoln, 2 I-90133 Palermo, Italy [email protected] Printed by Luxograph s.r.l., Piazza Bartolomeo da Messina, 2/E - Palermo Registration at Tribunale di Palermo, no. 27 of 12 July 1991 ISSN: 1120-4052 printed, 2240-4538 online DOI: 10.7320/FlMedit26.001 Copyright © by International Foundation pro Herbario Mediterraneo, Palermo Contents V. Hugonnot & L. Chavoutier: A modern record of one of the rarest European mosses, Ptychomitrium incurvum (Ptychomitriaceae), in Eastern Pyrenees, France . 5 P. Chène, M. -

Folegandros Island (Kiklades, Greece)
EDINBURGH JOURNAL OF BOTANY 72 ( 3 ): 391 – 412 (2015) 391 © Trustees of the Royal Botanic Garden Edinburgh (2015) doi:10.1017/S0960428615000128 CONTRIBUTION TO THE FLORA AND BIOGEOGRAPHY OF THE KIKLADES: FOLEGANDROS ISLAND (KIKLADES, GREECE) K . K OUGIOUMOUTZIS , A . T INIAKOU , O . G EORGIOU & T . G EORGIADIS The island of Folegandros, located between the Milos and Santorini archipelagos in the southern Kiklades (Greece), constitutes together with Ios and Sikinos the south-central part of the phytogeographical region of the Kiklades. Its flora consists of 474 taxa, 47 of which are under statutory protection, 40 are Greek endemics and 145 are reported here for the first time. We show that Folegandros has the highest percentage of Greek endemics in the phytogeographical area of the Kiklades. The known distribution of the endemic Muscari cycladicum subsp. cycladicum is expanded, being reported for the first time outside the South Aegean Volcanic Arc. The floristic cross-correlation between Folegandros and other parts of the phytogeographical region of the Kiklades by means of Sørensen’s index revealed that its phytogeographical affinities are stronger to Anafi Island than to any other part of the Kiklades. Keywords . Aegean flora , biodiversity , endemism , phytogeography . I NTRODUCTION The Aegean Sea has long attracted the attention of biogeographers (Turrill, 1929 ; Strid, 1996 ), since it is characterised by high environmental and topographical hetero- geneity (Blondel et al. , 2010 ), diversity and endemism (Strid, 1996 ). The Aegean archipelago consists of more than 7000 islands and islets (Triantis & Mylonas, 2009 ), most of which are located in the phytogeographical region of the Kiklades. Intensive field work has taken place in this region, which is characterised as one of the most floristically explored phytogeographical regions of Greece (Dimopoulos et al. -

Thomas Coulter's Californian Exsiccata
Aliso: A Journal of Systematic and Evolutionary Botany Volume 37 Issue 1 Issue 1–2 Article 2 2019 Plantae Coulterianae: Thomas Coulter’s Californian Exsiccata Gary D. Wallace California Botanic Garden, Claremont, CA Follow this and additional works at: https://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Wallace, Gary D. (2020) "Plantae Coulterianae: Thomas Coulter’s Californian Exsiccata," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 37: Iss. 1, Article 2. Available at: https://scholarship.claremont.edu/aliso/vol37/iss1/2 Aliso, 37(1–2), pp. 1–73 ISSN: 0065-6275 (print), 2327-2929 (online) PLANTAE COULTERIANAE: THOMAS COULTER’S CALIFORNIAN EXSICCATA Gary D. Wallace California Botanic Garden [formerly Rancho Santa Ana Botanic Garden], 1500 North College Avenue, Claremont, California 91711 ([email protected]) abstract An account of the extent, diversity, and importance of the Californian collections of Thomas Coulter in the herbarium (TCD) of Trinity College, Dublin, Ireland, is presented here. It is based on examination of collections in TCD, several other collections available online, and referenced literature. Additional infor- mation on historical context, content of herbarium labels and annotations is included. Coulter’s collections in TCD are less well known than partial duplicate sets at other herbaria. He was the first botanist to cross the desert of southern California to the Colorado River. Coulter’s collections in TCD include not only 60 vascular plant specimens previously unidentified as type material but also among the first moss andmarine algae specimens known to be collected in California. A list of taxa named for Thomas Coulter is included. -

Interpretation Manual of European Union Habitats - EUR27 Is a Scientific Reference Document
INTERPRETATION MANUAL OF EUROPEAN UNION HABITATS EUR 27 July 2007 EUROPEAN COMMISSION DG ENVIRONMENT Nature and biodiversity The Interpretation Manual of European Union Habitats - EUR27 is a scientific reference document. It is based on the version for EUR15, which was adopted by the Habitats Committee on 4. October 1999 and consolidated with the new and amended habitat types for the 10 accession countries as adopted by the Habitats Committee on 14 March 2002 with additional changes for the accession of Bulgaria and Romania as adopted by the Habitats Committee on 13 April 2007 and for marine habitats to follow the descriptions given in “Guidelines for the establishment of the Natura 2000 network in the marine environment. Application of the Habitats and Birds Directives” published in May 2007 by the Commission services. A small amendment to Habitat type 91D0 was adopted by the Habitats Committee in its meeting on 14th October 2003. TABLE OF CONTENTS WHY THIS MANUAL? 3 HISTORICAL REVIEW 3 THE MANUAL 4 THE EUR15 VERSION 5 THE EUR25 VERSION 5 THE EUR27 VERSION 6 EXPLANATORY NOTES 7 COASTAL AND HALOPHYTIC HABITATS 8 OPEN SEA AND TIDAL AREAS 8 SEA CLIFFS AND SHINGLE OR STONY BEACHES 17 ATLANTIC AND CONTINENTAL SALT MARSHES AND SALT MEADOWS 20 MEDITERRANEAN AND THERMO-ATLANTIC SALTMARSHES AND SALT MEADOWS 22 SALT AND GYPSUM INLAND STEPPES 24 BOREAL BALTIC ARCHIPELAGO, COASTAL AND LANDUPHEAVAL AREAS 26 COASTAL SAND DUNES AND INLAND DUNES 29 SEA DUNES OF THE ATLANTIC, NORTH SEA AND BALTIC COASTS 29 SEA DUNES OF THE MEDITERRANEAN COAST 35 INLAND -

Echium L. (Boraginaceae) (Speciation/Selection/Perennial Growth/Woodiness/Founding Populations) UTA-REGINA B6HLE*, HARTMUT H
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 11740-11745, October 1996 Evolution Island colonization and evolution of the insular woody habit in Echium L. (Boraginaceae) (speciation/selection/perennial growth/woodiness/founding populations) UTA-REGINA B6HLE*, HARTMUT H. HILGER*, AND WILLIAM F. MARTINtt *Institut fur Systematische Botanik und Pflanzengeographie, Freie Universitat Berlin, Altensteinstrasse 6, D-14195 Berlin-Dahlem, Germany; and tInstitut fur Genetik, Technische Universitat Braunschweig, Spielmannstrasse 7, D-38023 Braunschweig, Germany Communicated by Peter H. Raven, Missouri Botanical Garden, St. Louis, MO, August 1, 1996 (received for review March 19, 1996) ABSTRACT Numerous island-inhabiting species of pre- mism is strict; no island species occur naturally in continental dominantly herbaceous angiosperm genera are woody shrubs floras and vice versa. Second, insular woodiness is very or trees. Such "insular woodiness" is strongly manifested in strongly pronounced in the genus; all but two of the island the genus Echium, in which the continental species of circum- species (Echium bonnetii and Echium pitardii) are woody mediterranean distribution are herbaceous, whereas endemic perennials, whereas the continental species are annual to species of islands along the Atlantic coast of north Africa are perennial herbs (or very rarely subshrubs). Third, the ages of woody perennial shrubs. The history of37 Echium species was the islands on which the woody species occur are known traced with 70 kb of noncoding DNA determined from both (9-14), so that the evolutionary process can be correlated to chloroplast and nuclear genomes. In all, 239 polymorphic geologic time. Island species of Echium reveal a degree of positions with 137 informative sites, in addition to 27 infor- morphological diversity comparable to that found in the mative indels, were found. -

(Rubiaceae), a Uniquely Distylous, Cleistogamous Species Eric (Eric Hunter) Jones
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2012 Floral Morphology and Development in Houstonia Procumbens (Rubiaceae), a Uniquely Distylous, Cleistogamous Species Eric (Eric Hunter) Jones Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] THE FLORIDA STATE UNIVERSITY COLLEGE OF ARTS AND SCIENCES FLORAL MORPHOLOGY AND DEVELOPMENT IN HOUSTONIA PROCUMBENS (RUBIACEAE), A UNIQUELY DISTYLOUS, CLEISTOGAMOUS SPECIES By ERIC JONES A dissertation submitted to the Department of Biological Science in partial fulfillment of the requirements for the degree of Doctor of Philosophy Degree Awarded: Summer Semester, 2012 Eric Jones defended this dissertation on June 11, 2012. The members of the supervisory committee were: Austin Mast Professor Directing Dissertation Matthew Day University Representative Hank W. Bass Committee Member Wu-Min Deng Committee Member Alice A. Winn Committee Member The Graduate School has verified and approved the above-named committee members, and certifies that the dissertation has been approved in accordance with university requirements. ii I hereby dedicate this work and the effort it represents to my parents Leroy E. Jones and Helen M. Jones for their love and support throughout my entire life. I have had the pleasure of working with my father as a collaborator on this project and his support and help have been invaluable in that regard. Unfortunately my mother did not live to see me accomplish this goal and I can only hope that somehow she knows how grateful I am for all she’s done. iii ACKNOWLEDGEMENTS I would like to acknowledge the members of my committee for their guidance and support, in particular Austin Mast for his patience and dedication to my success in this endeavor, Hank W. -

New Host-Plant Records and Description of Larval Habitats (Lepidoptera: Noctuidae)
© Münchner Ent. Ges., download www.biologiezentrum.at Mitt. Münch. Ent. Ges. 96 29-42 München, 30.09.2006 ISSN 0340-4943 Deserticolous Noctuidae from Israel: New host-plant records and description of larval habitats (Lepidoptera: Noctuidae) Vasiliy D. KRAVCHENKO, Axel HAUSMANN & Günter C. MÜLLER Abstract So far, the insect fauna of the deserts and semi-deserts of Israel is poorly investigated. In this paper, 30 host-plant records are presented for 16 deserticolous noctuid species from Israel. For nine species host- plant records are published here for the first time: Anumeta atrosignata (WALKER, 1858), Anumeta asiatica WILTSHIRE, 1961, Drasteria kabylaria (A. BANG-HAAS, 1906), Gnamptonyx innexa (WALKER, 1858), Anydrophila stuebeli (CALBERLA, 1891), Cucullia improba muelleri (HACKER, 2001), Caradrina casearia (STAUDINGER, 1900), Euxoa canariensis diamondi BOURSIN, 1940, Agrotis herzogi REBEL, 1911. Larvae of a new Odontelia species were found living subterraneously in unconsolidated sand dunes. Introduction Israel is located at the eastern side of the Mediterranean basin, at the northern end of the Syrian - East African Rift valley. In contrast to the more uniform and monotonous landscapes of the Levant, Israel is topographically distinct with a large variety of different habitats (KOSSWIG 1955). The northern part of Israel includes Mt. Hermon, 2.200 m above sea-level, with annual snow and typical Tragacanth vegetation, while the Dead Sea area in the center is about 400 m below sea-level with Ethiopian pockets rich in Afro-tropical fauna and flora surrounded by hyper-arid deserts that stretch to the south (BYTINSKI- SALZ 1961; ZOHARY & ORSHANSKY 1949). The north and center of the country is Mediterranean while the south and east are predominantly arid, with almost two thirds of Israel being semi-desert and desert (ORNI & EFRAT 1980). -

Santa Monica Mountains National Recreation Area Vascular Plant
Santa Monica Mountains National Recreation Area Vascular Plant Species List (as derived from NPSpecies 18 Dec 2006) FAMILY NAME Scientific Name (Common Name) (* = non-native) - [Abundance] ASPLENIACEAE AIZOACEAE Asplenium vespertinum (spleenwort) - [Rare] Carpobrotus edulis (hottentot-fig) * - [Common] Galenia pubescens * - [Rare] AZOLLACEAE Malephora crocea * - [Uncommon] Azolla filiculoides (duck fern, mosquito fern) - [Rare] Mesembryanthemum crystallinum (common ice plant) * - [Common] BLECHNACEAE Mesembryanthemum nodiflorum (slender-leaved ice plant) * Woodwardia fimbriata (chain fern) - [Uncommon] - [Uncommon] DENNSTAEDTIACEAE Tetragonia tetragonioides (New Zealand-spinach) * - Pteridium aquilinum var. pubescens (western bracken) - [Uncommon] [Uncommon] AMARANTHACEAE DRYOPTERIDACEAE Amaranthus albus (tumbleweed) - [Common] Dryopteris arguta (coastal woodfern) - [Common] Amaranthus blitoides (prostrate pigweed) * - [Common] Amaranthus californicus (California amaranth) - [Uncommon] EQUISETACEAE Amaranthus deflexus (low amaranth) * - [Uncommon] Equisetum arvense - [Uncommon] Amaranthus powellii - [Unknown] Equisetum hyemale ssp. affine (common scouring rush) - Amaranthus retroflexus (rough pigweed) * - [Common] [Uncommon] Equisetum laevigatum (smooth scouring-rush) - [Uncommon] ANACARDIACEAE Equisetum telmateia ssp. braunii (giant horsetail) - Malosma laurina (laurel sumac) - [Common] [Uncommon] Rhus integrifolia (lemonadeberry) - [Common] Equisetum X ferrissi ((sterile hybrid)) - [Unknown] Rhus ovata (sugar -
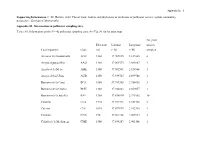
Supporting Information. C. M. Herrera. 2020. Flower Traits, Habitat, and Phylogeny As Predictors of Pollinator Service: a Plant Community Perspective
Appendix S1 – 1 Supporting Information. C. M. Herrera. 2020. Flower traits, habitat, and phylogeny as predictors of pollinator service: a plant community perspective. Ecological Monographs. Appendix S1. Information on pollinator sampling sites. TABLE S1. Information on the N = 42 pollinator sampling sites. See Fig. S1 for location map. No. plant Elevation Latitude Longitude species Local toponym Code (m) (º N) (º W) sampled Arenales del Guadalentín AGU 1360 37.909835 2.837405 4 Arroyo Aguaderillos AAG 1180 37.961573 2.883887 1 Arroyo de la Mesa AME 1100 37.902591 2.928966 1 Arroyo de los Ubios AUB 1250 37.939725 2.899766 1 Barranco de la Canal BCA 1580 37.789385 2.956438 3 Barranco de la Charca BCH 1360 37.942843 2.859057 1 Barranco de la Juanfría BJU 1380 37.836814 2.975302 10 Calarilla CLA 1710 37.944316 2.851708 2 Calerón CLE 1075 37.897973 2.942710 1 Cantalar CAN 770 37.964158 2.907974 3 Cañada de la Medianega CME 1580 37.894287 2.903146 1 Appendix S1 – 2 Cañada Pajarera CPA 1640 37.927456 2.805615 1 Collado del Calvario CCA 1420 37.950202 2.885484 1 Collado Zamora CZA 1420 37.839329 2.999615 2 Coto del Valle CVA 850 37.930542 2.928517 7 Cuesta del Bazar CBA 1330 37.906523 2.907470 1 Cuevas Bermejas CBE 1160 37.966959 2.851117 5 Fuente Bermejo FBE 1550 37.932268 2.840672 14 Fuente la Reina FRE 1460 37.941698 2.833632 2 Guadalentín GUA 1310 37.900404 2.836944 8 Hoyos de Muñoz HMU 1110 37.984831 2.876597 3 La Cabrilla LCA 1600 37.932364 2.780637 5 La Fresnedilla LFR 1080 37.980387 2.855875 2 La Mesa LME 1610 37.889823 2.913788 2 Las