I E •F AMPI-1 TAMI ES by a Thesis Submitted for the Degree of Doctor
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

(Mdma) in Non Conventional Matrices and Its Applications in Clinical Toxicology
Doctoral Thesis Doctorat Farmacología Departament de Farmacología, de Terapéutica i de Toxicologia DISTRIBUTION OF 3,4-METHYLENEDIOXYMETHAMPHETAMINE (MDMA) IN NON CONVENTIONAL MATRICES AND ITS APPLICATIONS IN CLINICAL TOXICOLOGY SIMONA PICHINI p Doctoral Thesis Doctorat Farmacología Departament de Farmacología, de Terapéutica i de Toxicologia DISTRIBUTION OF 3,4-METHYLENEDIOXYMETHAMPHETAMINE (MDMA) IN NON CONVENTIONAL MATRICES AND ITS APPLICATIONS IN CLINICAL TOXICOLOGY Doctoral thesis submitted by Simona Pichini as a partial fulfillment of the requirements for the degree of Doctor by the Universitat Autònoma de Barcelona. The studies included in this thesis have been realized under the direction of Dr. Rafael de la Torre Fornell and Dr. Magí Farré Albaladejo at the Pharmacology Unit of the Institut Municipal d'Investigació Mèdica (IMIM), Barcelona, Spain; and at the Drug Research and Control Department of the Istituto Superiore di Sanità, Roma, Italy. Doctorate Program of the Universitat Autònoma de Barcelona. Signature of Thesis Director Signature of Thesis Director (Dr. Magí Farré Albaladejo) (Dr. Rafael de la Torre Fornell) Signature of Doctorand (Simona Pichini) Yo soy yo y aquéllos a quienes amo Jorge Bucay To my friends, treasure of my life Acknowledgements Acknowledgements A number of people contributed to high extent to achieve the objectives of this Doctoral Thesis prepared between the two cities of Rome and Barcelona. This means that there are many people I’d the opportunity to meet, to work with, to share wonderful and terrible moments, and that I’d like to thank in these pages. First of all, to Dr. Piergiorgio Zuccaro, my “creator”, who always believed in me, supported my job and hardly fought to give me all the possible opportunities to grow up as an investigator; To Dr. -

Metabolomics Analysis Reveals Large Effects of Gut Microflora on Mammalian Blood Metabolites
Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites William R. Wikoffa, Andrew T. Anforab, Jun Liub, Peter G. Schultzb,1, Scott A. Lesleyb, Eric C. Petersb, and Gary Siuzdaka,1 aDepartment of Molecular Biology and Center for Mass Spectrometry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037; and bGenomics Institute of the Novartis Research Foundation, San Diego, CA 92121 Communicated by Steve A. Kay, University of California at San Diego, La Jolla, CA, December 19, 2008 (received for review December 12, 2008) Although it has long been recognized that the enteric community of found to extract energy from their food more efficiently compared bacteria that inhabit the human distal intestinal track broadly impacts with lean counterparts due to alterations in the composition of their human health, the biochemical details that underlie these effects gut microflora that resulted in an increased complement of genes remain largely undefined. Here, we report a broad MS-based metabo- for polysaccharide metabolism (10). It has also been observed that lomics study that demonstrates a surprisingly large effect of the gut bile salt hydrolase encoding genes are enriched in the gut micro- ‘‘microbiome’’ on mammalian blood metabolites. Plasma extracts biome, and that enteric bacteria carry out a wide range of bile acid from germ-free mice were compared with samples from conventional modifications (6, 14). These metagenomic studies suggest that the (conv) animals by using various MS-based methods. Hundreds of metabolites derived from this diverse microbial community can features were detected in only 1 sample set, with the majority of have a direct role in human health and disease. -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Poisons Act.Fm
LAWS OF BRUNEI CHAPTER 114 POISONS Enactment No. 13 of 1956 Amended by S 103/1958 Enactment No. 6 of 1967 S 101/1979 1984 Edition, Chapter 114 Amended by S 16/1996 S 28/2001 GN 273/2002 REVISED EDITION 2015 B.L.R.O. 1/2015 LAWS OF BRUNEI Poisons CAP. 114 1 LAWS OF BRUNEI REVISED EDITION 2015 CHAPTER 114 POISONS ARRANGEMENT OF SECTIONS Section 1. Citation. 2. Interpretation. 3. Description of poisons. 4. Licensing Officers. 5. General prohibition with respect to importation and sale of poisons. 6. Prohibitions and provisions relating to sale of poisons. 7. Exemptions in respect of medicines. 8. Exemptions in respect of sale. 9. Possession of poisons. 10. Issue of licences. 11. Different kinds of licence. 12. Licences to be numbered and registered. 13. Annual list to be published. 14. Forms of licence. 15. Search and search warrants. 16. Powers of exemption. 17. Penalties. B.L.R.O. 1/2015 LAWS OF BRUNEI 2 CAP. 114 Poisons 18. Jurisdiction. 19. Prosecutions. 20. Prohibition of sale to persons under 18. 21. Rules. SCHEDULE — POISONS LIST ____________________________ LAWS OF BRUNEI Poisons CAP. 114 3 POISONS ACT An Act to regulate the importation, possession, manufacture, compounding, storage, transport and sale of poisons Commencement: 1st January 1983 [S 61/1957] Citation. 1. This Act may be cited as the Poisons Act. Interpretation. 2. In this Act, unless the context otherwise requires — “dentist” means a dentist licensed under the Medical Practitioners and Dentists Act (Chapter 112) and includes a Government dentist; “export”, in relation -
![Metabolism of [IC ]Methamphetamine in Man, the Guinea Pig and the Rat](https://docslib.b-cdn.net/cover/0260/metabolism-of-ic-methamphetamine-in-man-the-guinea-pig-and-the-rat-640260.webp)
Metabolism of [IC ]Methamphetamine in Man, the Guinea Pig and the Rat
Biochem. J. (1972) 129, 11-22 11 Printed in Great Britain Metabolism of [IC ]Methamphetamine in Man, the Guinea Pig and the Rat By J. CALDWELL, L. G. DRING and R. T. WILLIAMS Department of Biochemistry, St. Mary's Hospital Medical School, London W.2, U.K. (Received 2 March 1972) 1. The metabolites of (±)-2-methylamino-1-phenyl[1-14C]propane ([14C]methamphet- amine) in urine were examined in man, rat and guinea pig. 2. In two male human subjects receiving the drug orally (20mg per person) about 90% ofthe 14C was excreted in the urine in 4 days. The urine ofthe first day was examined for metabolites, and the main metabolites were the unchanged drug (22% of the dose) and 4-hydroxymethamphetamine (15 %). Minor metabolites were hippuric acid, norephedrine, 4-hydroxyamphetamine, 4-hydroxy- norephedrine and an acid-labile precursor of benzyl methyl ketone. 3. In the rat some 82 % of the dose of '4C (45mg/kg) was excreted in the urine and 2-3 % in the faeces in 3-4 days. In 2 days the main metabolites in the urine were 4-hydroxymethamphetamine (31 % ofdose), 4-hydroxynorephedrine (16 %) and unchanged drug (11 %). Minor metabo- lites were amphetamine, 4-hydroxyamphetamine and benzoic acid. 4. The guinea pig was injected intraperitoneally with the drug at two doses, 10 and 45mg/kg. In both cases nearly 90% of the 14C was excreted, mainly in the urine after the lower dose, but in the urine (69%) and faeces (18%) after the higher dose. The main metabolites in the guinea pig were benzoic acid and its conjugates. -
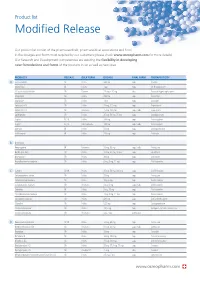
Modified Release
Product list Modified Release Our product list consist of the pharmaceuticals, pharmaceutical associations and food in the dosages and forms most required by our customers (please check www.osmopharm.com for more details). Our Research and Development competencies are assuring the flexibility in developing new formulations and forms of the products in list as well as new ones. PRODUCTS RELEASE BULK FORM DOSAGE FINAL FORM THERAPEUTICITY A Acetazolamide SR Pellets 500 mg caps Diuretic Alfacalcidol IR Pellets 1 μg caps Vit D supplement Alfuzosin Hydrochloride SR Powder 2.5 mg – 10 mg tabs Prostatic Hypertrophy agent Allopurinol SR Pellets 300 mg caps Antiurolytic Alprazolam SR Pellets 1 mg caps Anxiolytic Ambroxol HCI SR Pellets 75 mg, 120 mg caps Expectorant Ambroxol HCI SR Resinates 75 mg, 120 mg caps / tabs Expectorant Amitriptyline SR Pellets 25 mg, 50 mg, 75 mg caps Antidepressant Aspirin EC, IR Pellets 100 mg caps Anticoagulant Aspirin EC, IR Microcapsules 100 mg caps / tabs Anticoagulant Atenolol IR Pellets 50 mg caps Antihypertensive Azithromycin IR Pellets 250 mg caps Antibiotic B B-complex Benproperine IR Resinates 25 mg, 50 mg caps / tabs Antitussive Betahistine 2HCI SR Pellets 12 mg, 24 mg, 48 mg caps Vasodilator Bromopride* SR Pellets 20 mg caps Antiemetic Brompheniramine maleate SR Pellets 6 mg, 8 mg, 12 mg caps Antihistaminic C Caffeine SR, IR Pellets 25 mg, 50 mg, 300 mg caps CNS Stimulant Carbetapentane citrate SR Pellets 75 mg caps Antitussive Carbinoxamine maleate SR Pellets 4mg, 6 mg caps Antihistaminic Carbinoxamine -

Pdf 36.06 Kb
PROJECT REVIEW “Synthesis and Characterisation of Metabolites for the Integration in a Comprehensive Screening Procedure utilizing LC-MS/MS” W. Schänzer, M. Parr (German Sport University, Cologne, Germany) In the fight against doping the laboratories are confronted with an increasing number of substances to screen on. Therefore new methods have to be implemented by the laboratories. To keep the costs for doping control analysis acceptable, to ensure rapid reporting times and to lower the amount of urine needed to screen for all substances, a comprehensive screening for different classes of substances is desirable. Following the 2005 application WADA has granted a pilot project to check for the applicability of direct LC-MS/MS measurement of sulfoconjugates of heavy volatile stimulants. As most of the beta-2 agonists and heavy volatilestimulants are conjugated to sulfuric acid in humans an extension of the method to other compounds is desirable. As reference substances of the conjugates are barely available, during the pilot project the sulfoconjugates of p-Hydroxyamphetamine, p-Hydroxymetamphetamine (Pholedrine), p- Hydroxyephedrine, p-Hydroxynorephedrine, Etilefrine and Etamivan were synthesized by coupling the aglycons to sulfuric acid by reaction with sulfur trioxide pyridine complex. The objective of the continuation is to extend the combined screening procedure for diuretics and heavy volatile stimulants developed in the pilot project to other compounds. Thus, studies on the metabolism have to be reviewed and relevant metabolites have to be synthesised. The structures of all relevant products will be confirmed by nuclear magnetic resonance. For the integration in a comprehensive screening procedure the analytes will be characterised by means of LC-MS/MS. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

KI-311UR-IMM Vab Methamphetamine Urine HEIA
HANDLE ALL URINE SPECIMENS AS IF THEY ARE POTENTIALLY INFECTIOUS. Catalog Number: 311UR-0025 25 mL Kit Catalog Number: 311UR-0100 100 mL Kit ASSAY PROCEDURE Catalog Number: 311UR-0500 500 mL Kit Analyzers capable of maintaining a constant temperature, pipetting samples and Catalog Number: 311UR-0060W 60 mL Kit reagents, mixing reagents, timing the reaction accurately and measuring enzymatic Intended Use: rates at 340 nm can be used to perform the assay. The assay has been validated for The Immunalysis Methamphetamine Urine Enzyme Immunoassay is a homogeneous use with the Olympus AU400e, the following Beckman Coulter analyzers: AU480, enzyme immunoassay with a dual cutoff of 500ng/mL and 1000ng/mL. The assay is AU680, AU2700, AU5400 and AU5800. intended for use in laboratories for the qualitative and semi-quantitative analysis of Methamphetamine in human urine with automated clinical chemistry analyzers. This assay Refer to the analyzer-specific Application Sheet which contains parameters for use. is calibrated against Methamphetamine. This in-vitro diagnostic device is for prescription QUALITY CONTROL AND CALIBRATION use only. Good laboratory practice suggests the use of control specimens to ensure assay The semi-quantitative mode is for purposes of enabling laboratories to determine an performance. Control results must fall within established ranges determined by your appropriate dilution of the specimen for confirmation by a confirmatory method such as laboratory. QC materials should be used in accordance with local, state and/or federal Gas Chromatography/ Mass Spectrometry (GC-MS) or permitting laboratories to establish regulations or accreditation requirements. quality control procedures. For a qualitative analysis use either the 500ng/mL or 1000ng/mL calibrator as a cutoff The Immunalysis Methamphetamine Urine Enzyme Immunoassay Kit provides only level to distinguish “positive” and “negative” specimens. -

HIPPURIC ACID in Urine 8300
HIPPURIC ACID in urine 8300 C6H5CONHCH2COOH MW: 179.18 CAS: 495-69-2 RTECS: MR8150000 METHOD: 8300, Issue 2 EVALUATION: PARTIAL Issue 1: 15 February 1984 Issue 2: 15 August 1994 BIOLOGICAL INDICATOR OF: exposure to toluene. SYNONYMS: N-benzoylglycine SAMPLING MEASUREMENT SPECIMEN: urine, end of shift after 2 days exposure TECHNIQUE: VISIBLE ABSORPTION SPECTROPHOTOMETRY VOLUME: 50 to 100 mL in 125-mL plastic bottle ANALYTE: complex of hippuric acid PRESERVATIVE: a few crystals of thymol; keep at about and benzenesulfonyl chloride 4 °C WAVELENGTH: 410 nm SHIPMENT: pack in insulated shipper with bagged refrigerant; ship by air express PATH LENGTH: 1 cm SAMPLE CALIBRATION: aqueous hippuric acid standards STABILITY: stable 1 day @ 20 °C; 1 week @ 4 C; and 2 months @ •20 C QUALITY CONTROLS: frozen pooled urine; correct for creatinine content CONTROLS: collect pre-shift urines as well as urines from non-exposed controls RANGE: 0.005 to 0.5 g/L (1:5 urine dilution) ESTIMATED LOD: 0.002 g/L PRECISION (S r): 0.06 ACCURACY: not determined APPLICABILITY: Toluene is metabolized by the body and is excreted in the urine as hippuric acid, the glycine congugate of benzoic acid. This method is useful in screening workers exposed to toluene in the absence of xylene or styrene. The lat ter 2 compounds produce metabolites that are measured as "hippuric acid." INTERFERENCES: In addition to positive interferences from styrene and xylene in the workplace, the ingestion of sodium benzoate in food, salicylic acid, or aspirin by the worker will produce a positive interference. A careful work history/ questionnaire is suggested. -

Or Methionine, for Detoxication Mechanisms
GROWTH-INHIBITION PRODUCED IN RATS BY THE ORAL ADMINISTRATION OF SODIUM BENZOATE EFFECTS OF VARIOUS DIETARY SUPPLEMENTS* ABRAHAM WHITE Growth inhibition in rats has been produced by the incorpora- tion of a variety of toxic compounds into the diet.2" 22, 23, Further, it has been possible to demonstrate that stimulation of growth occurs under these experimental conditions when one of the sulfur-contain- ing amino acids, cysteine, cystine, or methionine, is superimposed on the basal diet still containing the growth-inhibitory compound. Under these conditions the animals resume growth at a rate com- parable to that observed on the basal diet alone. In addition, other substances, e.g., glutathione and cystine disulfoxide, which are known to stimulate growth in rats stunted by one of the so-called "cystine- deficient" diets, have also been shown to be effective growth stimu- lators when added to the basal, growth-inhibiting diets. Inasmuch as these growth-promoting dietary supplements exert their action when injected subcutaneously, as well as following oral administra- tion, it has been concluded that their effect is a true metabolic one and not due to a reaction occurring in the intestine prior to absorption. The specific and unique capacity of the sulfur-containing amino acids, and of substances behaving in nutrition experiments in a man- ner similar to these amino acids, to stimulate the growth of animals ingesting a growth-inhibitory basal diet has led to the suggestion that the inhibition of the growth of the rat by certain organic compounds "is a manifestation of the production of a specific deficiency in the sulfur-containing amino acids, probably by imposing on the organism an increased requirement for organic sulfur, in the form of cystine or methionine, for detoxication mechanisms."' However, although direct proof for the formation of a detoxication product (mercap- turic acid) is clearly established for the monohalogen benzenes, and * From the Department of Physiological Chemistry, Yale University School of Medicine. -

NIDA Drug Supply Program Catalog, 25Th Edition
RESEARCH RESOURCES DRUG SUPPLY PROGRAM CATALOG 25TH EDITION MAY 2016 CHEMISTRY AND PHARMACEUTICS BRANCH DIVISION OF THERAPEUTICS AND MEDICAL CONSEQUENCES NATIONAL INSTITUTE ON DRUG ABUSE NATIONAL INSTITUTES OF HEALTH DEPARTMENT OF HEALTH AND HUMAN SERVICES 6001 EXECUTIVE BOULEVARD ROCKVILLE, MARYLAND 20852 160524 On the cover: CPK rendering of nalfurafine. TABLE OF CONTENTS A. Introduction ................................................................................................1 B. NIDA Drug Supply Program (DSP) Ordering Guidelines ..........................3 C. Drug Request Checklist .............................................................................8 D. Sample DEA Order Form 222 ....................................................................9 E. Supply & Analysis of Standard Solutions of Δ9-THC ..............................10 F. Alternate Sources for Peptides ...............................................................11 G. Instructions for Analytical Services .........................................................12 H. X-Ray Diffraction Analysis of Compounds .............................................13 I. Nicotine Research Cigarettes Drug Supply Program .............................16 J. Ordering Guidelines for Nicotine Research Cigarettes (NRCs)..............18 K. Ordering Guidelines for Marijuana and Marijuana Cigarettes ................21 L. Important Addresses, Telephone & Fax Numbers ..................................24 M. Available Drugs, Compounds, and Dosage Forms ..............................25