Poisons Act.Fm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Mdma) in Non Conventional Matrices and Its Applications in Clinical Toxicology
Doctoral Thesis Doctorat Farmacología Departament de Farmacología, de Terapéutica i de Toxicologia DISTRIBUTION OF 3,4-METHYLENEDIOXYMETHAMPHETAMINE (MDMA) IN NON CONVENTIONAL MATRICES AND ITS APPLICATIONS IN CLINICAL TOXICOLOGY SIMONA PICHINI p Doctoral Thesis Doctorat Farmacología Departament de Farmacología, de Terapéutica i de Toxicologia DISTRIBUTION OF 3,4-METHYLENEDIOXYMETHAMPHETAMINE (MDMA) IN NON CONVENTIONAL MATRICES AND ITS APPLICATIONS IN CLINICAL TOXICOLOGY Doctoral thesis submitted by Simona Pichini as a partial fulfillment of the requirements for the degree of Doctor by the Universitat Autònoma de Barcelona. The studies included in this thesis have been realized under the direction of Dr. Rafael de la Torre Fornell and Dr. Magí Farré Albaladejo at the Pharmacology Unit of the Institut Municipal d'Investigació Mèdica (IMIM), Barcelona, Spain; and at the Drug Research and Control Department of the Istituto Superiore di Sanità, Roma, Italy. Doctorate Program of the Universitat Autònoma de Barcelona. Signature of Thesis Director Signature of Thesis Director (Dr. Magí Farré Albaladejo) (Dr. Rafael de la Torre Fornell) Signature of Doctorand (Simona Pichini) Yo soy yo y aquéllos a quienes amo Jorge Bucay To my friends, treasure of my life Acknowledgements Acknowledgements A number of people contributed to high extent to achieve the objectives of this Doctoral Thesis prepared between the two cities of Rome and Barcelona. This means that there are many people I’d the opportunity to meet, to work with, to share wonderful and terrible moments, and that I’d like to thank in these pages. First of all, to Dr. Piergiorgio Zuccaro, my “creator”, who always believed in me, supported my job and hardly fought to give me all the possible opportunities to grow up as an investigator; To Dr. -

Muscarinic Acetylcholine Receptor
mAChR Muscarinic acetylcholine receptor mAChRs (muscarinic acetylcholine receptors) are acetylcholine receptors that form G protein-receptor complexes in the cell membranes of certainneurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibersin the parasympathetic nervous system. mAChRs are named as such because they are more sensitive to muscarine than to nicotine. Their counterparts are nicotinic acetylcholine receptors (nAChRs), receptor ion channels that are also important in the autonomic nervous system. Many drugs and other substances (for example pilocarpineand scopolamine) manipulate these two distinct receptors by acting as selective agonists or antagonists. Acetylcholine (ACh) is a neurotransmitter found extensively in the brain and the autonomic ganglia. www.MedChemExpress.com 1 mAChR Inhibitors & Modulators (+)-Cevimeline hydrochloride hemihydrate (-)-Cevimeline hydrochloride hemihydrate Cat. No.: HY-76772A Cat. No.: HY-76772B Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic receptor agonist, is a candidate therapeutic drug for receptor agonist, is a candidate therapeutic drug for xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR The general pharmacol. properties of this drug on the The general pharmacol. properties of this drug on the gastrointestinal, urinary, and reproductive systems and other… gastrointestinal, urinary, and reproductive systems and other… Purity: >98% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 10mM x 1mL in DMSO, Size: 10mM x 1mL in DMSO, 1 mg, 5 mg 1 mg, 5 mg AC260584 Aclidinium Bromide Cat. No.: HY-100336 (LAS 34273; LAS-W 330) Cat. -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Pharmacy and Poisons Act 1979
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS ACT 1979 1979 : 26 TABLE OF CONTENTS PART I PRELIMINARY 1 Short title 2 Interpretation PART II THE PHARMACY COUNCIL 3 The Pharmacy Council 4 Membership of the Council 4A Functions of the Council 4B Protection from personal liability 4C Annual Report 5 Proceedings of the Council, etc PART III REGISTRATION OF PHARMACISTS 6 Offence to practise pharmacy if not registered 7 Registration as a pharmacist 7A Re-registration as non-practising member 7AA Period of validity of registration 8 Code of Conduct 9 Pharmacy Profession Complaints Committee 10 Investigation of complaint by Committee 10A Inquiry into complaint by Council 10B Inquiry by Council of its own initiative 11 Surrender of registration 12 Restoration of name to register 1 PHARMACY AND POISONS ACT 1979 13 Proof of registration 14 Appeals 14A Fees 14B Amendment of Seventh Schedule 15 Regulations for this part PART IV REGISTRATION OF PHARMACIES 16 Register of pharmacies 17 Registration of premises as registered pharmacies 18 Unfit premises: new applications 19 Unfit premises: registered pharmacies 20 Appeals 21 When certificates of unfitness take effect 22 Regulations for this Part PART V CONTROL OF PRESCRIPTIONS AND IMPORTATION 23 Prescriptions to be in a certain form 23A Validity of a prescription 24 Supply by registered pharmacist of equivalent medicines 25 Restrictions on the importation of medicines 26 Declaration relating to imported medicines [repealed] PART VI CONTROL OF DRUGS 27 Certain substances to be sold on prescription -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -
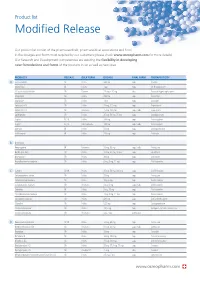
Modified Release
Product list Modified Release Our product list consist of the pharmaceuticals, pharmaceutical associations and food in the dosages and forms most required by our customers (please check www.osmopharm.com for more details). Our Research and Development competencies are assuring the flexibility in developing new formulations and forms of the products in list as well as new ones. PRODUCTS RELEASE BULK FORM DOSAGE FINAL FORM THERAPEUTICITY A Acetazolamide SR Pellets 500 mg caps Diuretic Alfacalcidol IR Pellets 1 μg caps Vit D supplement Alfuzosin Hydrochloride SR Powder 2.5 mg – 10 mg tabs Prostatic Hypertrophy agent Allopurinol SR Pellets 300 mg caps Antiurolytic Alprazolam SR Pellets 1 mg caps Anxiolytic Ambroxol HCI SR Pellets 75 mg, 120 mg caps Expectorant Ambroxol HCI SR Resinates 75 mg, 120 mg caps / tabs Expectorant Amitriptyline SR Pellets 25 mg, 50 mg, 75 mg caps Antidepressant Aspirin EC, IR Pellets 100 mg caps Anticoagulant Aspirin EC, IR Microcapsules 100 mg caps / tabs Anticoagulant Atenolol IR Pellets 50 mg caps Antihypertensive Azithromycin IR Pellets 250 mg caps Antibiotic B B-complex Benproperine IR Resinates 25 mg, 50 mg caps / tabs Antitussive Betahistine 2HCI SR Pellets 12 mg, 24 mg, 48 mg caps Vasodilator Bromopride* SR Pellets 20 mg caps Antiemetic Brompheniramine maleate SR Pellets 6 mg, 8 mg, 12 mg caps Antihistaminic C Caffeine SR, IR Pellets 25 mg, 50 mg, 300 mg caps CNS Stimulant Carbetapentane citrate SR Pellets 75 mg caps Antitussive Carbinoxamine maleate SR Pellets 4mg, 6 mg caps Antihistaminic Carbinoxamine -

Pdf 36.06 Kb
PROJECT REVIEW “Synthesis and Characterisation of Metabolites for the Integration in a Comprehensive Screening Procedure utilizing LC-MS/MS” W. Schänzer, M. Parr (German Sport University, Cologne, Germany) In the fight against doping the laboratories are confronted with an increasing number of substances to screen on. Therefore new methods have to be implemented by the laboratories. To keep the costs for doping control analysis acceptable, to ensure rapid reporting times and to lower the amount of urine needed to screen for all substances, a comprehensive screening for different classes of substances is desirable. Following the 2005 application WADA has granted a pilot project to check for the applicability of direct LC-MS/MS measurement of sulfoconjugates of heavy volatile stimulants. As most of the beta-2 agonists and heavy volatilestimulants are conjugated to sulfuric acid in humans an extension of the method to other compounds is desirable. As reference substances of the conjugates are barely available, during the pilot project the sulfoconjugates of p-Hydroxyamphetamine, p-Hydroxymetamphetamine (Pholedrine), p- Hydroxyephedrine, p-Hydroxynorephedrine, Etilefrine and Etamivan were synthesized by coupling the aglycons to sulfuric acid by reaction with sulfur trioxide pyridine complex. The objective of the continuation is to extend the combined screening procedure for diuretics and heavy volatile stimulants developed in the pilot project to other compounds. Thus, studies on the metabolism have to be reviewed and relevant metabolites have to be synthesised. The structures of all relevant products will be confirmed by nuclear magnetic resonance. For the integration in a comprehensive screening procedure the analytes will be characterised by means of LC-MS/MS. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

KI-311UR-IMM Vab Methamphetamine Urine HEIA
HANDLE ALL URINE SPECIMENS AS IF THEY ARE POTENTIALLY INFECTIOUS. Catalog Number: 311UR-0025 25 mL Kit Catalog Number: 311UR-0100 100 mL Kit ASSAY PROCEDURE Catalog Number: 311UR-0500 500 mL Kit Analyzers capable of maintaining a constant temperature, pipetting samples and Catalog Number: 311UR-0060W 60 mL Kit reagents, mixing reagents, timing the reaction accurately and measuring enzymatic Intended Use: rates at 340 nm can be used to perform the assay. The assay has been validated for The Immunalysis Methamphetamine Urine Enzyme Immunoassay is a homogeneous use with the Olympus AU400e, the following Beckman Coulter analyzers: AU480, enzyme immunoassay with a dual cutoff of 500ng/mL and 1000ng/mL. The assay is AU680, AU2700, AU5400 and AU5800. intended for use in laboratories for the qualitative and semi-quantitative analysis of Methamphetamine in human urine with automated clinical chemistry analyzers. This assay Refer to the analyzer-specific Application Sheet which contains parameters for use. is calibrated against Methamphetamine. This in-vitro diagnostic device is for prescription QUALITY CONTROL AND CALIBRATION use only. Good laboratory practice suggests the use of control specimens to ensure assay The semi-quantitative mode is for purposes of enabling laboratories to determine an performance. Control results must fall within established ranges determined by your appropriate dilution of the specimen for confirmation by a confirmatory method such as laboratory. QC materials should be used in accordance with local, state and/or federal Gas Chromatography/ Mass Spectrometry (GC-MS) or permitting laboratories to establish regulations or accreditation requirements. quality control procedures. For a qualitative analysis use either the 500ng/mL or 1000ng/mL calibrator as a cutoff The Immunalysis Methamphetamine Urine Enzyme Immunoassay Kit provides only level to distinguish “positive” and “negative” specimens. -

( 12 ) United States Patent
US010131766B2 (12 ) United States Patent (10 ) Patent No. : US 10 , 131, 766 B2 Hazen et al. (45 ) Date of Patent: Nov . 20 , 2018 ( 54 ) UNSATURATED POLYESTER RESIN 6 ,619 ,886 B1 9 /2003 Harrington 6 ,692 , 802 B1 2 / 2004 Nava SYSTEM FOR CURED IN - PLACE PIPING 7 , 135 ,087 B2 11/ 2006 Blackmore et al. 7 ,799 ,228 B2 9 /2010 Bomak et al . (71 ) Applicant : Interplastic Corporation , Saint Paul, 8 , 047, 238 B2 11/ 2011 Wiessner et al . MN (US ) 8 ,053 ,031 B2 11/ 2011 Stanley et al. 8 ,092 ,689 B2 1 / 2012 Gosselin (72 ) Inventors : Benjamin R . Hazen , Roseville , MN 8 , 298 , 360 B2 10 / 2012 Da Silveira et al. 8 ,418 , 728 B1 4 / 2013 Kiest , Jr . (US ) ; David J . Herzog , Maple Grove , 8 ,586 ,653 B2 11 / 2013 Klopsch et al. MN (US ) ; Louis R . Ross, Cincinnati , 8 ,636 , 869 B2 1 / 2014 Wiessner et al . OH (US ) ; Joel R . Weber , Moundsview , 8 ,741 , 988 B2 6 /2014 Klopsch et al. MN (US ) 8 , 877 , 837 B2 11/ 2014 Yu et al. 9 ,068 , 045 B2 6 /2015 Nava et al. 9 ,074 , 040 B2 7 / 2015 Turshani et al. ( 73 ) Assignee : Interplastic Corporation , St. Paul , MN 9 , 150 , 709 B2 10 / 2015 Klopsch et al . (US ) 9 , 207 , 155 B1 12 / 2015 Allouche et al. 9 ,273 ,815 B2 3 / 2016 Gillanders et al. ( * ) Notice : Subject to any disclaimer, the term of this 9 , 371, 950 B2 6 / 2016 Hairston et al. patent is extended or adjusted under 35 9 , 550 , 933 B2 1 /2017 Chatterji et al . -

NIDA Drug Supply Program Catalog, 25Th Edition
RESEARCH RESOURCES DRUG SUPPLY PROGRAM CATALOG 25TH EDITION MAY 2016 CHEMISTRY AND PHARMACEUTICS BRANCH DIVISION OF THERAPEUTICS AND MEDICAL CONSEQUENCES NATIONAL INSTITUTE ON DRUG ABUSE NATIONAL INSTITUTES OF HEALTH DEPARTMENT OF HEALTH AND HUMAN SERVICES 6001 EXECUTIVE BOULEVARD ROCKVILLE, MARYLAND 20852 160524 On the cover: CPK rendering of nalfurafine. TABLE OF CONTENTS A. Introduction ................................................................................................1 B. NIDA Drug Supply Program (DSP) Ordering Guidelines ..........................3 C. Drug Request Checklist .............................................................................8 D. Sample DEA Order Form 222 ....................................................................9 E. Supply & Analysis of Standard Solutions of Δ9-THC ..............................10 F. Alternate Sources for Peptides ...............................................................11 G. Instructions for Analytical Services .........................................................12 H. X-Ray Diffraction Analysis of Compounds .............................................13 I. Nicotine Research Cigarettes Drug Supply Program .............................16 J. Ordering Guidelines for Nicotine Research Cigarettes (NRCs)..............18 K. Ordering Guidelines for Marijuana and Marijuana Cigarettes ................21 L. Important Addresses, Telephone & Fax Numbers ..................................24 M. Available Drugs, Compounds, and Dosage Forms ..............................25