Frangula Alnus) Have Allelopathic Effects On
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Systematikss11 8.Pdf
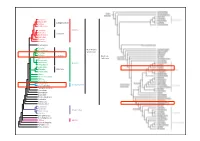
Asterales Dipsacales Campanulanae Apiales Aquifoliales Lamiales Asteridae Solanales Lamianae Gentianales Garryales Ericales Cornales Saxifragales Fagales Kern-Eudico- Cucurbitales tyledoneae Rosales Fabales Fabanae Eudicoty- Zygophyllales ledoneae Celastrales Oxalidales Rosidae Malpighiales Sapindales Malvales Malvanae Brassicales Vitales Crossosomatales Myrtales Geraniales Polygonales Caryophyllidae Caryophyllales Berberidopsidales Santalales Gunnerales Buxaceae Trochodendrales Proteales Sabiaceae Ranunculales Canellales Piperales Magnoliidae Magnoliales Laurales Chloanthaceae Ceratophyllaceae Liliidae Liliidae Austrobaileyales Nymphaeales Amborellales Klasse: Magnoliopsida (Angiospermae) Dicotyledoneae, Zweikeimblättrige Ordnung: Rosales Familie: Rhamnaceae (Kreuzdorngewächse) Merkmale: – Holzpflanzen mit wechselständigen oder gegenständigen, ungeteilten Blättern mit krautigen oder dornigen Nebenblättern – radiäre, kleine, gelblich-grüne, meist zwittrige Blüten – meist 5-zählige, selten 4-zählige Blüten – epipetal (= vor den Kronblättern stehende) Staubblätter – intrastaminaler Diskus – Perianth perigyn = mittelständiger Fruchtknoten, selten unterständig – Steinfrüchte, Nussfrüchte oder Spaltfrüchte Klasse: Magnoliopsida (Angiospermae) Dicotyledoneae, Zweikeimblättrige Ordnung: Rosales Familie: Rhamnaceae (Kreuzdorngewächse) Rhamnus frangula = Frangula alnus (Faulbaum) Kelchblatt Kronblatt Merkmale: – 5-zählige Blüte Staubblatt – Rinde mit grauweißen Korkwarzen (= Lentizellen) – Blätter elliptisch-eiförmig, ganzrandig – beerenähnliche -

Invasion of Transition Hardwood Forests by Exotic Rhamnus Frangula: Chronology and Site Requirements
University of New Hampshire University of New Hampshire Scholars' Repository Master's Theses and Capstones Student Scholarship Spring 2007 Invasion of transition hardwood forests by exotic Rhamnus frangula: Chronology and site requirements Hanna S. Wingard University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/thesis Recommended Citation Wingard, Hanna S., "Invasion of transition hardwood forests by exotic Rhamnus frangula: Chronology and site requirements" (2007). Master's Theses and Capstones. 286. https://scholars.unh.edu/thesis/286 This Thesis is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Master's Theses and Capstones by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. INVASION OF TRANSITION HARDWOOD FORESTS BY EXOTIC RHAMNUS FRANGULA: CHRONOLOGY AND SITE REQUIREMENTS BY HANNA S. WINGARD Baccalaureate Degree, University of Michigan, 2000 THESIS Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of M aster of Science in Natural Resources May, 2007 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: 1443642 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. -

Glossy Buckthorn Rhamnusoriental Frangula Bittersweet / Frangula Alnus Control Guidelines Fact Sheet
Glossy buckthorn Oriental bittersweet Rhamnus frangula / Frangula alnus Control Guidelines Fact Sheet NH Department of Agriculture, Markets & Food, Division of Plant Industry, 29 Hazen Dr, Concord, NH 03301 (603) 271-3488 Common Name: Glossy buckthorn Latin Name: Rhamnus frangula / Frangula alnus New Hampshire Invasive Species Status: Prohibited (Agr 3800) Native to: Japan leaves (spring) Glossy buckthorn invasion Sapling (summer) Flowers (spring) Roadside invasion of saplings Fleshy fruits (fall) Gray bark w/ lenticels (Summer) Emodin in berries - effects to birds Fall foliage (Autumn) Description: Deciduous shrub or small tree measuring 20' by 15'. Bark: Grayish to brown with raised lenticels. Stems: Cinnamon colored with light gray lenticels. Leaves: Alternate, simple and broadly ovate. Flowers: Inconspicuous, 4- petaled, greenish-yellow, mid-May. Fruit: Fleshy, 1/4” diameter turning black in the fall. Zone: 3-7. Habitat: Adapts to most conditions including pH, heavy shade to full sun. Spread: Seeds are bird dispersed. Comments: Highly Aggressive, fast growing, outcompetes native species. Controls: Remove seedlings and saplings by hand. Larger trees can be cut or plants can be treated with an herbicide. General Considerations Glossy buckthorn can either grow as a multi-stemmed shrub or single-stemmed tree up to 23’ (7 m) tall. Leaves are deciduous, simple, and generally arranged alternately. Leaves are dark-green and glossy above while dull-green below. The leaf margins are smooth/entire and tend to be slightly wavy. Flowers are small, about ¼” and somewhat inconspicuous forming in May to June. They develop and in small clusters of 2-8. Fruits form in mid to late summer and contain 2-3 seeds per berry. -

FLORA from FĂRĂGĂU AREA (MUREŞ COUNTY) AS POTENTIAL SOURCE of MEDICINAL PLANTS Silvia OROIAN1*, Mihaela SĂMĂRGHIŢAN2
ISSN: 2601 – 6141, ISSN-L: 2601 – 6141 Acta Biologica Marisiensis 2018, 1(1): 60-70 ORIGINAL PAPER FLORA FROM FĂRĂGĂU AREA (MUREŞ COUNTY) AS POTENTIAL SOURCE OF MEDICINAL PLANTS Silvia OROIAN1*, Mihaela SĂMĂRGHIŢAN2 1Department of Pharmaceutical Botany, University of Medicine and Pharmacy of Tîrgu Mureş, Romania 2Mureş County Museum, Department of Natural Sciences, Tîrgu Mureş, Romania *Correspondence: Silvia OROIAN [email protected] Received: 2 July 2018; Accepted: 9 July 2018; Published: 15 July 2018 Abstract The aim of this study was to identify a potential source of medicinal plant from Transylvanian Plain. Also, the paper provides information about the hayfields floral richness, a great scientific value for Romania and Europe. The study of the flora was carried out in several stages: 2005-2008, 2013, 2017-2018. In the studied area, 397 taxa were identified, distributed in 82 families with therapeutic potential, represented by 164 medical taxa, 37 of them being in the European Pharmacopoeia 8.5. The study reveals that most plants contain: volatile oils (13.41%), tannins (12.19%), flavonoids (9.75%), mucilages (8.53%) etc. This plants can be used in the treatment of various human disorders: disorders of the digestive system, respiratory system, skin disorders, muscular and skeletal systems, genitourinary system, in gynaecological disorders, cardiovascular, and central nervous sistem disorders. In the study plants protected by law at European and national level were identified: Echium maculatum, Cephalaria radiata, Crambe tataria, Narcissus poeticus ssp. radiiflorus, Salvia nutans, Iris aphylla, Orchis morio, Orchis tridentata, Adonis vernalis, Dictamnus albus, Hammarbya paludosa etc. Keywords: Fărăgău, medicinal plants, human disease, Mureş County 1. -

Frangula Paruensis, a New Name for Rhamnus Longipes Steyermark (Rhamnaceae)
FRANGULA PARUENSIS, A NEW NAME FOR RHAMNUS LONGIPES STEYERMARK (RHAMNACEAE) GERARDO A. AYMARD C.1, 2 Abstract. The new name Frangula paruensis (Rhamnaceae) is proposed to replace the illegitimate homonym Rhamnus longipes Steyermark (1988). Chorological, taxonomic, biogeographical, and habitat notes about this taxon also are provided. Resumen. Se propone Frangula paruensis (Rhamnaceae) como un nuevo nombre para reemplazar el homónimo ilegítimo Rhamnus longipes Steyermark (1988). Se incluye información corológica, taxonómica, biogeográfica, y de hábitats acerca de la especie. Keywords: Frangula, Rhamnus, Rhamnaceae, Parú Massif, Tepuis flora, Venezuela Rhamnus L. and Frangula Miller (Rhamnaceae) have Frangula paruensis is a shrub, ca. 2 m tall, with leaves ca. 150 and ca. 50 species, respectively (Pool, 2013, 2015). ovate, or oblong-ovate, margin subrevolute, repand- These taxa are widely distributed around the world but are crenulate, a slightly elevated tertiary venation on the lower absent in Madagascar, Australia, and Polynesia (Medan and surface, and mature fruiting peduncle and pedicels 1–1.5 Schirarend, 2004). According to Grubov (1949), Kartesz cm long, and fruiting calyx lobes triangular-lanceolate and Gandhi (1994), Bolmgren and Oxelman (2004), and (two main features to separate Frangula from Rhamnus). Pool (2013) the recognition of Frangula is well supported. This species is endemic to the open, rocky savannas On the basis of historical and recent molecular work the on tepui slopes and summits at ca. 2000 m (Steyermark genus is characterized by several remarkable features. Pool and Berry, 2004). This Venezuelan taxon was described (2013: 448, table 1) summarized 11 features to separate the as Rhamnus longipes by Steyermark (1988), without two genera. -

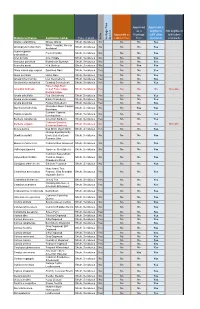
Herb Other Names Re Co Rde D Me Dicinal Us E Re
RECORDED USE RECORDED USE MEDICINAL US AROMATHERA IN COSMETICS IN RECORDED RECORDED FOOD USE FOOD IN Y E P HERB OTHER NAMES COMMENTS Parts Used Medicinally Abelmoschus moschatus Hibiscus abelmoschus, Ambrette, Musk mallow, Muskseed No No Yes Yes Abies alba European silver fir, silver fir, Abies pectinata Yes No Yes Yes Leaves & resin Abies balsamea Balm of Gilead, balsam fir Yes No Yes Yes Leaves, bark resin & oil Abies canadensis Hemlock spruce, Tsuga, Pinus bark Yes No No No Bark Abies sibirica Fir needle, Siberian fir Yes No Yes Yes Young shoots This species not used in aromatherapy but Abies Sibirica, Abies alba Miller, Siberian Silver Fir Abies spectabilis Abies webbiana, Himalayan silver fir Yes No No No Essential Oil are. Leaves Aqueous bark extract which is often concentrated and dried to produce a flavouring. Distilled with Extract, bark, wood, Acacia catechu Black wattle, Black catechu Yes Yes No No vodka to make Blavod (black vodka). flowering tops and gum Acacia farnesiana Cassie, Prickly Moses Yes Yes Yes Yes Ripe seeds pressed for cooking oil Bark, flowers Source of Gum Arabic (E414) and Guar Gum (E412), controlled miscellaneous food additive. Used Acacia senegal Guar gum, Gum arabic No Yes No Yes in foods as suspending and emulsifying agent. Acanthopanax senticosus Kan jang Yes No No No Kan Jang is a combination of Andrographis Paniculata and Acanthopanax Senticosus. Flavouring source including essential oil. Contains natural toxin thujone/thuyone whose levels in flavourings are limited by EU (Council Directive 88/388/EEC) and GB (SI 1992 No.1971) legislation. There are several chemotypes of Yarrow Essential Oil, which is steam distilled from the dried herb. -

Section 317.1A (16, 2)
1 WEEDS, §317.1A 317.1A Noxious weeds. 1. The following weeds are hereby declared to be noxious and shall be divided into two classes, as follows: a. Primary noxious weeds, which shall include: (1) Quack grass (Elymus repens). (2) Perennial sow thistle (Sonchus arvensis). (3) Canada thistle (Cirsium arvense). (4) Bull thistle (Cirsium vulgare). (5) European morning glory or field bindweed (Convolvulus arvensis). (6) Horse nettle (Solanum carolinense). (7) Leafy spurge (Euphorbia esula). (8) Perennial pepper-grass (Cardaria draba). (9) Russian knapweed (Acroptilon repens). (10) Buckthorn (Rhamnus spp., not to include Frangula alnus, syn. Rhamnus frangula). (11) All other species of thistles belonging in the genera of Cirsium and Carduus. (12) Palmer amaranth (Amaranthus palmeri). b. Secondary noxious weeds, which shall include: (1) Butterprint (Abutilon theophrasti) annual. (2) Cocklebur (Xanthium strumarium) annual. (3) Wild mustard (Sinapis arvensis) annual. (4) Wild carrot (Daucus carota) biennial. (5) Buckhorn (Plantago lanceolata) perennial. (6) Sheep sorrel (Rumex acetosella) perennial. (7) Sour dock (Rumex crispus) perennial. (8) Smooth dock (Rumex altissimus) perennial. (9) Poison hemlock (Conium maculatum). (10) Multiflora rose (Rosa multiflora). (11) Wild sunflower (wild strain of Helianthus annuus L.) annual. (12) Puncture vine (Tribulus terrestris) annual. (13) Teasel (Dipsacus spp.) biennial. (14) Shattercane (Sorghum bicolor) annual. 2. a. The multiflora rose (Rosa multiflora) shall not be considered a secondary noxious weed when cultivated for or used as understock for cultivated roses or as ornamental shrubs in gardens, or in any county whose board of supervisors has by resolution declared it not to be a noxious weed. b. Shattercane (Sorghum bicolor) shall not be considered a secondary noxious weed when cultivated or in any county whose board of supervisors has by resolution declared it not to be a noxious weed. -

An Assessment of Autumn Olive in Northern U.S. Forests Research Note NRS-204
United States Department of Agriculture An Assessment of Autumn Olive in Northern U.S. Forests Research Note NRS-204 This publication is part of a series of research notes that provide an overview of the invasive plant species monitored on an extensive systematic network of plots measured by the Forest Inventory and Analysis (FIA) program of the U.S. Forest Service, Northern Research Station (NRS). Each research note features one of the invasive plants monitored on forested plots by NRS FIA in the 24 states of the midwestern and northeastern United States. Background and Characteristics Autumn olive (Elaeagnus umbellata), a shrub of the Oleaster family (Elaeagnaceae), is native to eastern Asia and arrived in the United States in the 1830s. This vigorous invader was promoted for wildlife, landscaping, and erosion control. Tolerant of poor quality sites and full sun, it was often used for mine reclamation. Autumn olive disrupts native plant communities that require infertile soil by changing soil fertility through fixing nitrogen. Where it establishes, it can form dense thickets that shade out native plants (Czarapata 2005, Kaufman and Kaufman 2007, Kurtz 2013). Aside from the negative impact, autumn olive has important culinary and medicinal properties (Fordham et al. 2001, Guo et al. 2009). Figure 1.—Autumn olive flowers. Photo by Chris Evans, Description University of Illinois, from Bugwood.org, 1380001. Growth: woody, perennial shrub to 20 feet, often multi- stemmed; simple, alternate leaves with slightly wavy margins, green upper leaf surfaces, and silvery bottoms; shrubs leaf out early in the spring and retain leaves late in the fall. -

Extended Leaf Phenology and the Autumn Niche in Deciduous Forest Invasions
LETTER doi:10.1038/nature11056 Extended leaf phenology and the autumn niche in deciduous forest invasions Jason D. Fridley1 The phenology of growth in temperate deciduous forests, including development, biweekly leaf production and chlorophyll (Chl) content, the timing of leaf emergence and senescence, has strong control over and monthly photosynthetic rate on select leaves at a range of light ecosystem properties such as productivity1,2 and nutrient cycling3,4, levels (50–800 mmol pm22 s21). Although not all species were mea- and has an important role in the carbon economy of understory sured each year, a similar number of native and non-native species plants5–7. Extended leaf phenology, whereby understory species were monitored annually and data sets for most species involved at assimilate carbon in early spring before canopy closure or in late least 2 years (Supplementary Table 1). autumn after canopy fall, has been identified as a key feature of The timing of leaf emergence across species was sensitive to inter- many forest species invasions5,8–10, but it remains unclear whether annual variation in spring temperatures (Supplementary Fig. 1), with there are systematic differences in the growth phenology of native all stages of bud development occurring several weeks earlier in the and invasive forest species11 or whether invaders are more responsive warmer spring of 2010 (P , 0.001, year contrasts in Mann–Whitney to warming trends that have lengthened the duration of spring or U-tests adjusted for multiple testing; Fig. 1). However, the timing of autumn growth12. Here, in a 3-year monitoring study of 43 native early stages of bud activity was not significantly different between and 30 non-native shrub and liana species common to deciduous native and non-native species for any year (Fig. -
Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Natural Resources
Plants That Cause Problems – “Invasive Species” DestinationOakland.com STOP! What is an Invasive Species? Imagine spring wildflowers covered with a mat of twisted green vines. This is a sign that Oakland County Offenders invasive plants have moved in and native species have moved out. Invasive plant species originate in other parts of the world and are introduced into to the United States through flower a variety of ways. Not all plants introduced from other countries become invasive. The term invasive species is reserved for plants that grow and reproduce rapidly, causing changes to the areas where they become established. Invasive species impact our health, our economy seed and the environment, and can cost the United States $120 billion annually! flower pod flower A Ticking Time Bomb Growing unnoticed at first, invasive species spread and cover the landscape. They change the seed pod fruit ecology, affecting wildlife communities and the soil. Over time, invasive plant species form a monoculture in which the only plant growing is the invasive plant. Plants with Super Powers Invasive species have super powers to alter the ecological balance of nature. They flourish seed because controls in their native lands do not exist here. The Nature Conservancy ranks invasive species as the second leading cause of species extinction worldwide. Negative Effects of Invasive Species • Changes in hydrology— seed dispersal wetlands dry out • Release of chemicals into the soil that inhibit Black Swallow-wort Cynanchum louiseae & the growth of other plants. This is known as Pale Swallow-wort Cynanchum rossicum Autumn Olive Elaeagnus umbellate Garlic Mustard Allaria periolata Allelopathy. -

Glossy Buckthorn Frangula Alnus
Invasive Species—Best Control Practices Michigan Department of Natural Resources Michigan Natural Features Inventory 2/2012 Glossy buckthorn Frangula alnus Glossy buckthorn is native to Eurasia but has been com- monly planted in this country as a hedge and for wildlife food and cover. It was widely recommended for conserva- tion plantings in the Midwest until its invasive tendencies became apparent; it creates dense thickets and out-com- petes native vegetation. Its fruit is widely dispersed by birds and small mammals. Glossy buckthorn, like many invasive shrubs, leafs out early in the spring and retains its leaves late into fall, increasing its energy production and shading out native plants. It is a particular pest on wet sites and poses a significant threat to Michigan’s rich prairie fens, as well as other wetland com- munities. It is also successful on many upland sites including old fields, roadsides and open woods. Glossy buckthorn is an alternate host for alfalfa mosaic virus and crown fungus, which causes oat rust disease. It has also been implicated as a possible host for the soybean aphid. It is widely distributed in some parts of the state, but is just beginning to appear in others. If it is caught early in its initial invasion, it may be eradicated completely. Robert Vidéki, Doronicum Kft., Bugwood.org Identification Flowers: Habit: Glossy buckthorn flowers Glossy buckthorn is a small tree or shrub with a spreading are tiny with five greenish- crown growing up to 6 m (20 ft) tall. Typically, it has multiple white petals, arranged in stems when young, and develops into a tree with a trunk clusters at the bases of the that may reach 25 cm (10 in) in diameter at maturity.