Screening of Underlying Genetic Biomarkers for Ankylosing Spondylitis
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

CD81 Antibody (C-Term) Purified Rabbit Polyclonal Antibody (Pab) Catalog # AP6631B
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 CD81 Antibody (C-term) Purified Rabbit Polyclonal Antibody (Pab) Catalog # AP6631B Specification CD81 Antibody (C-term) - Product Information Application WB, IHC-P, FC,E Primary Accession P60033 Reactivity Human, Mouse Host Rabbit Clonality Polyclonal Isotype Rabbit Ig Antigen Region 176-203 CD81 Antibody (C-term) - Additional Information Gene ID 975 Other Names CD81 antigen, 26 kDa cell surface protein TAPA-1, Target of the antiproliferative antibody 1, Tetraspanin-28, Tspan-28, Western blot analysis of CD81 Antibody CD81, CD81, TAPA1, TSPAN28 (C-term) (Cat. #AP6631b) in mouse kidney(lane 1) and cerebellum(lane 2) tissue Target/Specificity lysates (35ug/lane). CD81 (arrow) was This CD81 antibody is generated from detected using the purified Pab. rabbits immunized with a KLH conjugated synthetic peptide between 176-203 amino acids from the C-terminal region of human CD81. Dilution WB~~1:1000 IHC-P~~1:10~50 FC~~1:10~50 Format Purified polyclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is prepared by Saturated Ammonium Sulfate (SAS) precipitation followed by dialysis against PBS. Storage Maintain refrigerated at 2-8°C for up to 2 Formalin-fixed and paraffin-embedded weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw human brain tissue with CD81 Antibody cycles. (C-term), which was peroxidase-conjugated to the secondary antibody, followed by DAB Precautions staining. This data demonstrates the use of CD81 Antibody (C-term) is for research use this antibody for immunohistochemistry; Page 1/4 10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 only and not for use in diagnostic or clinical relevance has not been evaluated. -

Analysis of Gene Expression Data for Gene Ontology
ANALYSIS OF GENE EXPRESSION DATA FOR GENE ONTOLOGY BASED PROTEIN FUNCTION PREDICTION A Thesis Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Master of Science Robert Daniel Macholan May 2011 ANALYSIS OF GENE EXPRESSION DATA FOR GENE ONTOLOGY BASED PROTEIN FUNCTION PREDICTION Robert Daniel Macholan Thesis Approved: Accepted: _______________________________ _______________________________ Advisor Department Chair Dr. Zhong-Hui Duan Dr. Chien-Chung Chan _______________________________ _______________________________ Committee Member Dean of the College Dr. Chien-Chung Chan Dr. Chand K. Midha _______________________________ _______________________________ Committee Member Dean of the Graduate School Dr. Yingcai Xiao Dr. George R. Newkome _______________________________ Date ii ABSTRACT A tremendous increase in genomic data has encouraged biologists to turn to bioinformatics in order to assist in its interpretation and processing. One of the present challenges that need to be overcome in order to understand this data more completely is the development of a reliable method to accurately predict the function of a protein from its genomic information. This study focuses on developing an effective algorithm for protein function prediction. The algorithm is based on proteins that have similar expression patterns. The similarity of the expression data is determined using a novel measure, the slope matrix. The slope matrix introduces a normalized method for the comparison of expression levels throughout a proteome. The algorithm is tested using real microarray gene expression data. Their functions are characterized using gene ontology annotations. The results of the case study indicate the protein function prediction algorithm developed is comparable to the prediction algorithms that are based on the annotations of homologous proteins. -

Molecular and Physiological Basis for Hair Loss in Near Naked Hairless and Oak Ridge Rhino-Like Mouse Models: Tracking the Role of the Hairless Gene
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2006 Molecular and Physiological Basis for Hair Loss in Near Naked Hairless and Oak Ridge Rhino-like Mouse Models: Tracking the Role of the Hairless Gene Yutao Liu University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Life Sciences Commons Recommended Citation Liu, Yutao, "Molecular and Physiological Basis for Hair Loss in Near Naked Hairless and Oak Ridge Rhino- like Mouse Models: Tracking the Role of the Hairless Gene. " PhD diss., University of Tennessee, 2006. https://trace.tennessee.edu/utk_graddiss/1824 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Yutao Liu entitled "Molecular and Physiological Basis for Hair Loss in Near Naked Hairless and Oak Ridge Rhino-like Mouse Models: Tracking the Role of the Hairless Gene." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Life Sciences. Brynn H. Voy, Major Professor We have read this dissertation and recommend its acceptance: Naima Moustaid-Moussa, Yisong Wang, Rogert Hettich Accepted for the Council: Carolyn R. -

CD226 T Cells Expressing the Receptors TIGIT and Divergent Phenotypes of Human Regulatory
The Journal of Immunology Divergent Phenotypes of Human Regulatory T Cells Expressing the Receptors TIGIT and CD226 Christopher A. Fuhrman,*,1 Wen-I Yeh,*,1 Howard R. Seay,* Priya Saikumar Lakshmi,* Gaurav Chopra,† Lin Zhang,* Daniel J. Perry,* Stephanie A. McClymont,† Mahesh Yadav,† Maria-Cecilia Lopez,‡ Henry V. Baker,‡ Ying Zhang,x Yizheng Li,{ Maryann Whitley,{ David von Schack,x Mark A. Atkinson,* Jeffrey A. Bluestone,‡ and Todd M. Brusko* Regulatory T cells (Tregs) play a central role in counteracting inflammation and autoimmunity. A more complete understanding of cellular heterogeneity and the potential for lineage plasticity in human Treg subsets may identify markers of disease pathogenesis and facilitate the development of optimized cellular therapeutics. To better elucidate human Treg subsets, we conducted direct transcriptional profiling of CD4+FOXP3+Helios+ thymic-derived Tregs and CD4+FOXP3+Helios2 T cells, followed by comparison with CD4+FOXP32Helios2 T conventional cells. These analyses revealed that the coinhibitory receptor T cell Ig and ITIM domain (TIGIT) was highly expressed on thymic-derived Tregs. TIGIT and the costimulatory factor CD226 bind the common ligand CD155. Thus, we analyzed the cellular distribution and suppressive activity of isolated subsets of CD4+CD25+CD127lo/2 T cells expressing CD226 and/or TIGIT. We observed TIGIT is highly expressed and upregulated on Tregs after activation and in vitro expansion, and is associated with lineage stability and suppressive capacity. Conversely, the CD226+TIGIT2 population was associated with reduced Treg purity and suppressive capacity after expansion, along with a marked increase in IL-10 and effector cytokine production. These studies provide additional markers to delineate functionally distinct Treg subsets that may help direct cellular therapies and provide important phenotypic markers for assessing the role of Tregs in health and disease. -
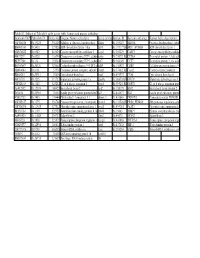
Table S3: Subset of Zebrafish Early Genes with Human And
Table S3: Subset of Zebrafish early genes with human and mouse orthologs Genbank ID(ZFZebrafish ID Entrez GenUnigene Name (zebrafish) Gene symbo Human ID Humann ortholog Human Gene description AW116838 Dr.19225 336425 Aldolase a, fructose-bisphosphate aldoa Hs.155247 ALDOA Fructose-bisphosphate aldola BM005100 Dr.5438 327026 ADP-ribosylation factor 1 like arf1l Hs.119177||HsARF1_HUMAN ADP-ribosylation factor 1 AW076882 Dr.6582 403025 Cancer susceptibility candidate 3 casc3 Hs.350229 CASC3 Cancer susceptibility candidat AI437239 Dr.6928 116994 Chaperonin containing TCP1, subun cct6a Hs.73072||Hs.CCT6A T-complex protein 1, zeta sub BE557308 Dr.134 192324 Chaperonin containing TCP1, subun cct7 Hs.368149 CCT7 T-complex protein 1, eta subu BG303647 Dr.26326 321602 Cyclin-dependent kinase 9 (CDC2-recdk9 Hs.150423 CDK9 Cell division protein kinase 9 AB040044 Dr.8161 57970 Coatomer protein complex, subunit zcopz1 Hs.37482||Hs.Copz2 Coatomer zeta-2 subunit BI888253 Dr.20911 30436 Eyes absent homolog 1 eya1 Hs.491997 EYA4 Eyes absent homolog 4 AI878758 Dr.3225 317737 Glutamate dehydrogenase 1a glud1a Hs.368538||HsGLUD1 Glutamate dehydrogenase 1, AW128619 Dr.1388 325284 G1 to S phase transition 1 gspt1 Hs.59523||Hs.GSPT1 G1 to S phase transition prote AF412832 Dr.12595 140427 Heat shock factor 2 hsf2 Hs.158195 HSF2 Heat shock factor protein 2 D38454 Dr.20916 30151 Insulin gene enhancer protein Islet3 isl3 Hs.444677 ISL2 Insulin gene enhancer protein AY052752 Dr.7485 170444 Pbx/knotted 1 homeobox 1.1 pknox1.1 Hs.431043 PKNOX1 Homeobox protein PKNOX1 -

A Rare Variant in MCF2L Identified Using Exclusion Linkage in A
European Journal of Human Genetics (2016) 24, 86–91 & 2016 Macmillan Publishers Limited All rights reserved 1018-4813/16 www.nature.com/ejhg ARTICLE A rare variant in MCF2L identified using exclusion linkage in a pedigree with premature atherosclerosis Stephanie Maiwald1,7, Mahdi M Motazacker1,7,8, Julian C van Capelleveen1, Suthesh Sivapalaratnam1, Allard C van der Wal2, Chris van der Loos2, John JP Kastelein1, Willem H Ouwehand3,4, G Kees Hovingh1, Mieke D Trip1,5, Jaap D van Buul6 and Geesje M Dallinga-Thie*,1 Cardiovascular disease (CVD) is a major cause of death in Western societies. CVD risk is largely genetically determined. The molecular pathology is, however, not elucidated in a large number of families suffering from CVD. We applied exclusion linkage analysis and next-generation sequencing to elucidate the molecular defect underlying premature CVD in a small pedigree, comprising two generations of which six members suffered from premature CVD. A total of three variants showed co-segregation with the disease status in the family. Two of these variants were excluded from further analysis based on the prevalence in replication cohorts, whereas a non-synonymous variant in MCF.2 Cell Line Derived Transforming Sequence-like protein (MCF2L, c.2066A4G; p.(Asp689Gly); NM_001112732.1), located in the DH domain, was only present in the studied family. MCF2L is a guanine exchange factor that potentially links pathways that signal through Rac1 and RhoA. Indeed, in HeLa cells, MCF2L689Gly failed to activate Rac1 as well as RhoA, resulting in impaired stress fiber formation. Moreover, MCF2L protein was found in human atherosclerotic lesions but not in healthy tissue segments. -

CD Markers Are Routinely Used for the Immunophenotyping of Cells
ptglab.com 1 CD MARKER ANTIBODIES www.ptglab.com Introduction The cluster of differentiation (abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules. So-called CD markers are routinely used for the immunophenotyping of cells. Despite this use, they are not limited to roles in the immune system and perform a variety of roles in cell differentiation, adhesion, migration, blood clotting, gamete fertilization, amino acid transport and apoptosis, among many others. As such, Proteintech’s mini catalog featuring its antibodies targeting CD markers is applicable to a wide range of research disciplines. PRODUCT FOCUS PECAM1 Platelet endothelial cell adhesion of blood vessels – making up a large portion molecule-1 (PECAM1), also known as cluster of its intracellular junctions. PECAM-1 is also CD Number of differentiation 31 (CD31), is a member of present on the surface of hematopoietic the immunoglobulin gene superfamily of cell cells and immune cells including platelets, CD31 adhesion molecules. It is highly expressed monocytes, neutrophils, natural killer cells, on the surface of the endothelium – the thin megakaryocytes and some types of T-cell. Catalog Number layer of endothelial cells lining the interior 11256-1-AP Type Rabbit Polyclonal Applications ELISA, FC, IF, IHC, IP, WB 16 Publications Immunohistochemical of paraffin-embedded Figure 1: Immunofluorescence staining human hepatocirrhosis using PECAM1, CD31 of PECAM1 (11256-1-AP), Alexa 488 goat antibody (11265-1-AP) at a dilution of 1:50 anti-rabbit (green), and smooth muscle KD/KO Validated (40x objective). alpha-actin (red), courtesy of Nicola Smart. PECAM1: Customer Testimonial Nicola Smart, a cardiovascular researcher “As you can see [the immunostaining] is and a group leader at the University of extremely clean and specific [and] displays Oxford, has said of the PECAM1 antibody strong intercellular junction expression, (11265-1-AP) that it “worked beautifully as expected for a cell adhesion molecule.” on every occasion I’ve tried it.” Proteintech thanks Dr. -

The VE-Cadherin/Amotl2 Mechanosensory Pathway Suppresses Aortic In�Ammation and the Formation of Abdominal Aortic Aneurysms
The VE-cadherin/AmotL2 mechanosensory pathway suppresses aortic inammation and the formation of abdominal aortic aneurysms Yuanyuan Zhang Karolinska Institute Evelyn Hutterer Karolinska Institute Sara Hultin Karolinska Institute Otto Bergman Karolinska Institute Maria Forteza Karolinska Institute Zorana Andonovic Karolinska Institute Daniel Ketelhuth Karolinska University Hospital, Stockholm, Sweden Joy Roy Karolinska Institute Per Eriksson Karolinska Institute Lars Holmgren ( [email protected] ) Karolinska Institute Article Keywords: arterial endothelial cells (ECs), vascular disease, abdominal aortic aneurysms Posted Date: June 15th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-600069/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License The VE-cadherin/AmotL2 mechanosensory pathway suppresses aortic inflammation and the formation of abdominal aortic aneurysms Yuanyuan Zhang1, Evelyn Hutterer1, Sara Hultin1, Otto Bergman2, Maria J. Forteza2, Zorana Andonovic1, Daniel F.J. Ketelhuth2,3, Joy Roy4, Per Eriksson2 and Lars Holmgren1*. 1Department of Oncology-Pathology, BioClinicum, Karolinska Institutet, Stockholm, Sweden. 2Department of Medicine Solna, BioClinicum, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden. 3Department of Cardiovascular and Renal Research, Institutet of Molecular Medicine, Univ. of Southern Denmark, Odense, Denmark 4Department of Molecular Medicine and Surgery, Karolinska Institutet, Karolinska University Hospital, Stockholm, -

NKTR-214 2017SITC Poster-P77
The Novel IL-2 Cytokine Immune Agonist NKTR-214 Harnesses the Adaptive and Innate Immune System for the Treatment of Solid Cancers Salah Eddine Bentebibel1, Chantale Bernatchez1, Cara Haymaker1, Michael Hurwitz3, Patrick Hwu1, Mario Sznol3, Nizar Tannir1, Anthony Conley1, Harriet Kluger3, Sandra Aung2, Michael Imperiale2, Mary Tagliaferri2, Christie Fanton2, Ernesto Iacucci2, Jonathan Zalevsky2, Ute Hoch2, Adi Diab1 1The University of Texas MD Anderson Cancer Center, Houston, TX, United States of America, 2Nektar Therapeutics, San Francisco, CA, United States of America, 3Yale School of Medicine, New Haven, CT, United States of America BACKGROUND RESULTS NKTR-214 Figure 1. NKTR-214 Mechanism of Action Figure 5. NKTR-214 induces activating markers and co-inhibitory Figure 10. Increase in T cell clonality in tumors after treatment • NKTR-214 is a CD122-biased cytokine agonist + NKTR-214 2-PEG 1-PEG receptors in proliferating CD4 T cells in peripheral blood with NKTR-214 conjugated with multiple releasable chains of (6-PEG) Active Cytokine Active Cytokine PD-1 CTLA-4 OX40 ICOS Irreversible Irreversible 15 40 20 80 5 polyethylene glycol (PEG) designed to provide Release Release • An increase in overall T cell n=11 n=15 n=9 n=15 4 (%CD4) sustained signaling through the heterodimeric + 30 60 3 (%CD4) 15 (%CD4) + (%CD4) + clonality observed in 8/12 + 10 + 2 IL-2 receptor pathway (IL-2R) to preferentially 20 1 PD-1 10 40 patients after NKTR-214 OX40 ICOS + + 10 + CTLA-4 LEGEND: + + 0 activate and expand effector CD8 T and NK 5 α Ki-67 treatment 1 NKTR-214 – Inactive 5 20 -1 Melanoma Ki67 Ki-67 5 Ki-67 + Ki-67 cells over Tregs (Figure 1). -

BD Pharmingen™ Purified Mouse Anti-CD247 (Py142)
BD Pharmingen™ Technical Data Sheet Purified Mouse anti-CD247 (pY142) Product Information Material Number: 558402 Alternate Name: CD3ζ Size: 0.1 mg Concentration: 0.5 mg/ml Clone: K25-407.69 Immunogen: Phosphorylated Human CD3ζ Peptide Isotype: Mouse IgG2a, κ Reactivity: QC Testing: Human Target MW: 21 kDa Storage Buffer: Aqueous buffered solution containing ≤0.09% sodium azide. Description The T cell receptor (TCR), expressed by thymocytes and T lymphocytes, is a multi-component cell-surface complex responsible for recognizing antigen in the context of MHC molecules. The antigen-specific binding component of the TCR, Ti, is a heterodimer of the variable Ig-like subunits a and b or g and d. Ti is non-covalently associated with an invariant set of molecules referred to as the CD3 polypeptides, g, d, e, and ζ. The CD3 z polypeptide (CD3z) was named CD247 at the 7th Human Leukocyte Differentiation Antigens Workshop. CD3 appears early in thymocyte differentiation and remains expressed on all mature T lymphocytes. After antigen recognition by the TCR, CD3z is the primary intracellular signal transducing subunit. It contains three ITAMs (Immunoreceptor Tyrosine-based Activation Motifs), each of which contains a pair of tyrosine residues that are phosphorylated by Lck and Fyn and are required for signal propagation. The molecular weight of CD3z is 16 kDa, and it is also observed as 32-kDa homodimers or as heterodimers with the g chain of Fc receptors. Upon phosphorylation, the CD3z monomer undergoes an apparent shift in electrophoretic mobility up to 21 kDa. The K25-407.69 monoclonal antibody recognizes the phosphorylated tyrosine 142 (pY142) in the third ITAM domain of human CD3z (CD247). -

CD81 Antibody Purified Mouse Monoclonal Antibody (Mab) Catalog # Am8557b
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 CD81 Antibody Purified Mouse Monoclonal Antibody (Mab) Catalog # AM8557b Specification CD81 Antibody - Product Information Application WB, IHC,E Primary Accession P60033 Other Accession P60034 Reactivity Human, Rat Host Mouse Clonality monoclonal Isotype IgG1,k CD81 Antibody - Additional Information Gene ID 975 Other Names CD81 antigen, 26 kDa cell surface protein TAPA-1, Target of the antiproliferative antibody 1, Tetraspanin-28, Tspan-28, Anti-CD81 Antibody at 1:4000 dilution + rat CD81, CD81, TAPA1, TSPAN28 cerebellum whole cell lysate Lysates/proteins at 20 µg per lane. Secondary Goat Target/Specificity Anti-Mouse IgG, (H+L), Peroxidase This CD81 antibody is generated from a conjugated at 1/10000 dilution. Predicted mouse immunized with recombinant protein band size : 26 kDa Blocking/Dilution buffer: from the human region of human CD81. 5% NFDM/TBST. Dilution WB~~1:2000 IHC~~1:1000 Format Purified monoclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is purified through a protein G column, followed by dialysis against PBS. Storage Maintain refrigerated at 2-8°C for up to 2 weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. Precautions CD81 Antibody is for research use only and not for use in diagnostic or therapeutic Anti-CD81 Antibody at 1:4000 dilution + procedures. human testis whole cell lysate Lysates/proteins at 20 µg per lane. Secondary Goat Anti-Mouse IgG, (H+L), Page 1/4 10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 CD81 Antibody - Protein Information Peroxidase conjugated at 1/10000 dilution. -

Novel Targets of Apparently Idiopathic Male Infertility
International Journal of Molecular Sciences Review Molecular Biology of Spermatogenesis: Novel Targets of Apparently Idiopathic Male Infertility Rossella Cannarella * , Rosita A. Condorelli , Laura M. Mongioì, Sandro La Vignera * and Aldo E. Calogero Department of Clinical and Experimental Medicine, University of Catania, 95123 Catania, Italy; [email protected] (R.A.C.); [email protected] (L.M.M.); [email protected] (A.E.C.) * Correspondence: [email protected] (R.C.); [email protected] (S.L.V.) Received: 8 February 2020; Accepted: 2 March 2020; Published: 3 March 2020 Abstract: Male infertility affects half of infertile couples and, currently, a relevant percentage of cases of male infertility is considered as idiopathic. Although the male contribution to human fertilization has traditionally been restricted to sperm DNA, current evidence suggest that a relevant number of sperm transcripts and proteins are involved in acrosome reactions, sperm-oocyte fusion and, once released into the oocyte, embryo growth and development. The aim of this review is to provide updated and comprehensive insight into the molecular biology of spermatogenesis, including evidence on spermatogenetic failure and underlining the role of the sperm-carried molecular factors involved in oocyte fertilization and embryo growth. This represents the first step in the identification of new possible diagnostic and, possibly, therapeutic markers in the field of apparently idiopathic male infertility. Keywords: spermatogenetic failure; embryo growth; male infertility; spermatogenesis; recurrent pregnancy loss; sperm proteome; DNA fragmentation; sperm transcriptome 1. Introduction Infertility is a widespread condition in industrialized countries, affecting up to 15% of couples of childbearing age [1]. It is defined as the inability to achieve conception after 1–2 years of unprotected sexual intercourse [2].