A Tandem CD19/CD20 CAR Lentiviral Vector Drives On-Target and Off
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CD81 Antibody (C-Term) Purified Rabbit Polyclonal Antibody (Pab) Catalog # AP6631B
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 CD81 Antibody (C-term) Purified Rabbit Polyclonal Antibody (Pab) Catalog # AP6631B Specification CD81 Antibody (C-term) - Product Information Application WB, IHC-P, FC,E Primary Accession P60033 Reactivity Human, Mouse Host Rabbit Clonality Polyclonal Isotype Rabbit Ig Antigen Region 176-203 CD81 Antibody (C-term) - Additional Information Gene ID 975 Other Names CD81 antigen, 26 kDa cell surface protein TAPA-1, Target of the antiproliferative antibody 1, Tetraspanin-28, Tspan-28, Western blot analysis of CD81 Antibody CD81, CD81, TAPA1, TSPAN28 (C-term) (Cat. #AP6631b) in mouse kidney(lane 1) and cerebellum(lane 2) tissue Target/Specificity lysates (35ug/lane). CD81 (arrow) was This CD81 antibody is generated from detected using the purified Pab. rabbits immunized with a KLH conjugated synthetic peptide between 176-203 amino acids from the C-terminal region of human CD81. Dilution WB~~1:1000 IHC-P~~1:10~50 FC~~1:10~50 Format Purified polyclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is prepared by Saturated Ammonium Sulfate (SAS) precipitation followed by dialysis against PBS. Storage Maintain refrigerated at 2-8°C for up to 2 Formalin-fixed and paraffin-embedded weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw human brain tissue with CD81 Antibody cycles. (C-term), which was peroxidase-conjugated to the secondary antibody, followed by DAB Precautions staining. This data demonstrates the use of CD81 Antibody (C-term) is for research use this antibody for immunohistochemistry; Page 1/4 10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 only and not for use in diagnostic or clinical relevance has not been evaluated. -

CD226 T Cells Expressing the Receptors TIGIT and Divergent Phenotypes of Human Regulatory
The Journal of Immunology Divergent Phenotypes of Human Regulatory T Cells Expressing the Receptors TIGIT and CD226 Christopher A. Fuhrman,*,1 Wen-I Yeh,*,1 Howard R. Seay,* Priya Saikumar Lakshmi,* Gaurav Chopra,† Lin Zhang,* Daniel J. Perry,* Stephanie A. McClymont,† Mahesh Yadav,† Maria-Cecilia Lopez,‡ Henry V. Baker,‡ Ying Zhang,x Yizheng Li,{ Maryann Whitley,{ David von Schack,x Mark A. Atkinson,* Jeffrey A. Bluestone,‡ and Todd M. Brusko* Regulatory T cells (Tregs) play a central role in counteracting inflammation and autoimmunity. A more complete understanding of cellular heterogeneity and the potential for lineage plasticity in human Treg subsets may identify markers of disease pathogenesis and facilitate the development of optimized cellular therapeutics. To better elucidate human Treg subsets, we conducted direct transcriptional profiling of CD4+FOXP3+Helios+ thymic-derived Tregs and CD4+FOXP3+Helios2 T cells, followed by comparison with CD4+FOXP32Helios2 T conventional cells. These analyses revealed that the coinhibitory receptor T cell Ig and ITIM domain (TIGIT) was highly expressed on thymic-derived Tregs. TIGIT and the costimulatory factor CD226 bind the common ligand CD155. Thus, we analyzed the cellular distribution and suppressive activity of isolated subsets of CD4+CD25+CD127lo/2 T cells expressing CD226 and/or TIGIT. We observed TIGIT is highly expressed and upregulated on Tregs after activation and in vitro expansion, and is associated with lineage stability and suppressive capacity. Conversely, the CD226+TIGIT2 population was associated with reduced Treg purity and suppressive capacity after expansion, along with a marked increase in IL-10 and effector cytokine production. These studies provide additional markers to delineate functionally distinct Treg subsets that may help direct cellular therapies and provide important phenotypic markers for assessing the role of Tregs in health and disease. -

CD Markers Are Routinely Used for the Immunophenotyping of Cells
ptglab.com 1 CD MARKER ANTIBODIES www.ptglab.com Introduction The cluster of differentiation (abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules. So-called CD markers are routinely used for the immunophenotyping of cells. Despite this use, they are not limited to roles in the immune system and perform a variety of roles in cell differentiation, adhesion, migration, blood clotting, gamete fertilization, amino acid transport and apoptosis, among many others. As such, Proteintech’s mini catalog featuring its antibodies targeting CD markers is applicable to a wide range of research disciplines. PRODUCT FOCUS PECAM1 Platelet endothelial cell adhesion of blood vessels – making up a large portion molecule-1 (PECAM1), also known as cluster of its intracellular junctions. PECAM-1 is also CD Number of differentiation 31 (CD31), is a member of present on the surface of hematopoietic the immunoglobulin gene superfamily of cell cells and immune cells including platelets, CD31 adhesion molecules. It is highly expressed monocytes, neutrophils, natural killer cells, on the surface of the endothelium – the thin megakaryocytes and some types of T-cell. Catalog Number layer of endothelial cells lining the interior 11256-1-AP Type Rabbit Polyclonal Applications ELISA, FC, IF, IHC, IP, WB 16 Publications Immunohistochemical of paraffin-embedded Figure 1: Immunofluorescence staining human hepatocirrhosis using PECAM1, CD31 of PECAM1 (11256-1-AP), Alexa 488 goat antibody (11265-1-AP) at a dilution of 1:50 anti-rabbit (green), and smooth muscle KD/KO Validated (40x objective). alpha-actin (red), courtesy of Nicola Smart. PECAM1: Customer Testimonial Nicola Smart, a cardiovascular researcher “As you can see [the immunostaining] is and a group leader at the University of extremely clean and specific [and] displays Oxford, has said of the PECAM1 antibody strong intercellular junction expression, (11265-1-AP) that it “worked beautifully as expected for a cell adhesion molecule.” on every occasion I’ve tried it.” Proteintech thanks Dr. -

The VE-Cadherin/Amotl2 Mechanosensory Pathway Suppresses Aortic In�Ammation and the Formation of Abdominal Aortic Aneurysms
The VE-cadherin/AmotL2 mechanosensory pathway suppresses aortic inammation and the formation of abdominal aortic aneurysms Yuanyuan Zhang Karolinska Institute Evelyn Hutterer Karolinska Institute Sara Hultin Karolinska Institute Otto Bergman Karolinska Institute Maria Forteza Karolinska Institute Zorana Andonovic Karolinska Institute Daniel Ketelhuth Karolinska University Hospital, Stockholm, Sweden Joy Roy Karolinska Institute Per Eriksson Karolinska Institute Lars Holmgren ( [email protected] ) Karolinska Institute Article Keywords: arterial endothelial cells (ECs), vascular disease, abdominal aortic aneurysms Posted Date: June 15th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-600069/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License The VE-cadherin/AmotL2 mechanosensory pathway suppresses aortic inflammation and the formation of abdominal aortic aneurysms Yuanyuan Zhang1, Evelyn Hutterer1, Sara Hultin1, Otto Bergman2, Maria J. Forteza2, Zorana Andonovic1, Daniel F.J. Ketelhuth2,3, Joy Roy4, Per Eriksson2 and Lars Holmgren1*. 1Department of Oncology-Pathology, BioClinicum, Karolinska Institutet, Stockholm, Sweden. 2Department of Medicine Solna, BioClinicum, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden. 3Department of Cardiovascular and Renal Research, Institutet of Molecular Medicine, Univ. of Southern Denmark, Odense, Denmark 4Department of Molecular Medicine and Surgery, Karolinska Institutet, Karolinska University Hospital, Stockholm, -

NKTR-214 2017SITC Poster-P77
The Novel IL-2 Cytokine Immune Agonist NKTR-214 Harnesses the Adaptive and Innate Immune System for the Treatment of Solid Cancers Salah Eddine Bentebibel1, Chantale Bernatchez1, Cara Haymaker1, Michael Hurwitz3, Patrick Hwu1, Mario Sznol3, Nizar Tannir1, Anthony Conley1, Harriet Kluger3, Sandra Aung2, Michael Imperiale2, Mary Tagliaferri2, Christie Fanton2, Ernesto Iacucci2, Jonathan Zalevsky2, Ute Hoch2, Adi Diab1 1The University of Texas MD Anderson Cancer Center, Houston, TX, United States of America, 2Nektar Therapeutics, San Francisco, CA, United States of America, 3Yale School of Medicine, New Haven, CT, United States of America BACKGROUND RESULTS NKTR-214 Figure 1. NKTR-214 Mechanism of Action Figure 5. NKTR-214 induces activating markers and co-inhibitory Figure 10. Increase in T cell clonality in tumors after treatment • NKTR-214 is a CD122-biased cytokine agonist + NKTR-214 2-PEG 1-PEG receptors in proliferating CD4 T cells in peripheral blood with NKTR-214 conjugated with multiple releasable chains of (6-PEG) Active Cytokine Active Cytokine PD-1 CTLA-4 OX40 ICOS Irreversible Irreversible 15 40 20 80 5 polyethylene glycol (PEG) designed to provide Release Release • An increase in overall T cell n=11 n=15 n=9 n=15 4 (%CD4) sustained signaling through the heterodimeric + 30 60 3 (%CD4) 15 (%CD4) + (%CD4) + clonality observed in 8/12 + 10 + 2 IL-2 receptor pathway (IL-2R) to preferentially 20 1 PD-1 10 40 patients after NKTR-214 OX40 ICOS + + 10 + CTLA-4 LEGEND: + + 0 activate and expand effector CD8 T and NK 5 α Ki-67 treatment 1 NKTR-214 – Inactive 5 20 -1 Melanoma Ki67 Ki-67 5 Ki-67 + Ki-67 cells over Tregs (Figure 1). -

BD Pharmingen™ Purified Mouse Anti-CD247 (Py142)
BD Pharmingen™ Technical Data Sheet Purified Mouse anti-CD247 (pY142) Product Information Material Number: 558402 Alternate Name: CD3ζ Size: 0.1 mg Concentration: 0.5 mg/ml Clone: K25-407.69 Immunogen: Phosphorylated Human CD3ζ Peptide Isotype: Mouse IgG2a, κ Reactivity: QC Testing: Human Target MW: 21 kDa Storage Buffer: Aqueous buffered solution containing ≤0.09% sodium azide. Description The T cell receptor (TCR), expressed by thymocytes and T lymphocytes, is a multi-component cell-surface complex responsible for recognizing antigen in the context of MHC molecules. The antigen-specific binding component of the TCR, Ti, is a heterodimer of the variable Ig-like subunits a and b or g and d. Ti is non-covalently associated with an invariant set of molecules referred to as the CD3 polypeptides, g, d, e, and ζ. The CD3 z polypeptide (CD3z) was named CD247 at the 7th Human Leukocyte Differentiation Antigens Workshop. CD3 appears early in thymocyte differentiation and remains expressed on all mature T lymphocytes. After antigen recognition by the TCR, CD3z is the primary intracellular signal transducing subunit. It contains three ITAMs (Immunoreceptor Tyrosine-based Activation Motifs), each of which contains a pair of tyrosine residues that are phosphorylated by Lck and Fyn and are required for signal propagation. The molecular weight of CD3z is 16 kDa, and it is also observed as 32-kDa homodimers or as heterodimers with the g chain of Fc receptors. Upon phosphorylation, the CD3z monomer undergoes an apparent shift in electrophoretic mobility up to 21 kDa. The K25-407.69 monoclonal antibody recognizes the phosphorylated tyrosine 142 (pY142) in the third ITAM domain of human CD3z (CD247). -

CD81 Antibody Purified Mouse Monoclonal Antibody (Mab) Catalog # Am8557b
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 CD81 Antibody Purified Mouse Monoclonal Antibody (Mab) Catalog # AM8557b Specification CD81 Antibody - Product Information Application WB, IHC,E Primary Accession P60033 Other Accession P60034 Reactivity Human, Rat Host Mouse Clonality monoclonal Isotype IgG1,k CD81 Antibody - Additional Information Gene ID 975 Other Names CD81 antigen, 26 kDa cell surface protein TAPA-1, Target of the antiproliferative antibody 1, Tetraspanin-28, Tspan-28, Anti-CD81 Antibody at 1:4000 dilution + rat CD81, CD81, TAPA1, TSPAN28 cerebellum whole cell lysate Lysates/proteins at 20 µg per lane. Secondary Goat Target/Specificity Anti-Mouse IgG, (H+L), Peroxidase This CD81 antibody is generated from a conjugated at 1/10000 dilution. Predicted mouse immunized with recombinant protein band size : 26 kDa Blocking/Dilution buffer: from the human region of human CD81. 5% NFDM/TBST. Dilution WB~~1:2000 IHC~~1:1000 Format Purified monoclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is purified through a protein G column, followed by dialysis against PBS. Storage Maintain refrigerated at 2-8°C for up to 2 weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. Precautions CD81 Antibody is for research use only and not for use in diagnostic or therapeutic Anti-CD81 Antibody at 1:4000 dilution + procedures. human testis whole cell lysate Lysates/proteins at 20 µg per lane. Secondary Goat Anti-Mouse IgG, (H+L), Page 1/4 10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 CD81 Antibody - Protein Information Peroxidase conjugated at 1/10000 dilution. -
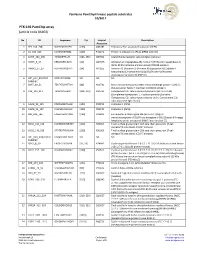
PTK-196 Pamchip Array (Article Code 86402)
PamGene PamChip4 kinase peptide substrates 10/2017 PTK-196 PamChip array (article code 86402) No ID Sequence Tyr Uniprot Description Accession 1 EFS_246_258 GGTDEGIYDVPLL [253] O43281 Embryonal Fyn-associated substrate (HEFS). 2 41_654_666 LDGENIYIRHSNL [660] P11171 Protein 4.1 (Band 4.1) (P4.1) (EPB4.1) (4.1R). 3 ACHD_383_395 YISKAEEYFLLKS [383, 390] Q07001 Acetylcholine receptor subunit delta precursor. 4 AMPE_5_17 EREGSKRYCIQTK [12] Q07075 Glutamyl aminopeptidase (EC 3.4.11.7) (EAP) (Aminopeptidase A) (APA) (Differentiation antigen gp160) (CD249 antigen). 5 ANXA2_17_29 HSTPPSAYGSVKA [24] P07355 Annexin A2 (Annexin-2) (Annexin II) (Lipocortin II) (Calpactin I heavychain) (Chromobindin-8) (p36) (Protein I) (Placental anticoagulantprotein IV) (PAP-IV). 6 ART_004_EAIYAAP EAIYAAPFAKKK NA NA NA FAKKKXC 7 B3AT_39_51 TEATATDYHTTSH [46] P02730 Band 3 anion transport protein (Anion exchange protein 1) (AE 1) (Solute carrier family 4 member 1) (CD233 antigen). 8 C1R_199_211 TEASGYISSLEYP [204, 210] P00736 Complement C1r subcomponent precursor (EC 3.4.21.41) (Complementcomponent 1, r subcomponent) [Contains: Complement C1r subcomponentheavy chain; Complement C1r subcomponent light chain]. 9 CALM_93_105 FDKDGNGYISAAE [100] P0DP23 Calmodulin (CaM). 10 CALM_95_107 KDGNGYISAAELR [100] P0DP23 Calmodulin (CaM). 11 CBL_693_705 EGEEDTEYMTPSS [700] P22681 E3 ubiquitin-protein ligase CBL (EC 6.3.2.-) (Signal transductionprotein CBL) (Proto-oncogene c-CBL) (Casitas B-lineage lymphoma proto-oncogene) (RING finger protein 55). 12 CD3Z_116_128 KDKMAEAYSEIGM [123] P20963 T-cell surface glycoprotein CD3 zeta chain precursor (T-cell receptorT3 zeta chain) (CD247 antigen). 13 CD3Z_146_158 STATKDTYDALHM [153] P20963 T-cell surface glycoprotein CD3 zeta chain precursor (T-cell receptorT3 zeta chain) (CD247 antigen). 14 ART_003_EAI(pY)AAP EAI(pY)AAPFAKKK NA NA NA FAKKKXC 15 CDK2_8_20 EKIGEGTYGVVYK [15, 19] P24941 Cyclin-dependent kinase 2 (EC:2.7.11.22) Cell division protein kinase 2 (EC 2.7.11.22) (p33 protein kinase). -

Is Associated with Autoimmune Diabetes and Correlates With
Variation in the Cd3ζ (Cd247) Gene Correlates with Altered T Cell Activation and Is Associated with Autoimmune Diabetes This information is current as Marie Lundholm, Sofia Mayans, Vinicius Motta, Anna of September 25, 2021. Löfgren-Burström, Jayne Danska and Dan Holmberg J Immunol 2010; 184:5537-5544; Prepublished online 16 April 2010; doi: 10.4049/jimmunol.0904012 http://www.jimmunol.org/content/184/10/5537 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2010/04/16/jimmunol.090401 Material 2.DC1 http://www.jimmunol.org/ References This article cites 51 articles, 21 of which you can access for free at: http://www.jimmunol.org/content/184/10/5537.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 25, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2010 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Variation in the Cd3z (Cd247) Gene Correlates with Altered T Cell Activation and Is Associated with Autoimmune Diabetes Marie Lundholm,* Sofia Mayans,*,† Vinicius Motta,*,‡,x Anna Lo¨fgren-Burstro¨m,* Jayne Danska,‡,x and Dan Holmberg*,† Tuning of TCR-mediated activation was demonstrated to be critical for lineage fate in T cell development, as well as in the control of autoimmunity. -

Angiogenic Properties of NK Cells in Cancer and Other Angiogenesis-Dependent Diseases
cells Review Angiogenic Properties of NK Cells in Cancer and Other Angiogenesis-Dependent Diseases Dorota M. Radomska-Le´sniewska 1, Agata Białoszewska 1,* and Paweł Kami ´nski 2 1 Center for Biostructure Research, Department of Histology and Embryology, Medical University of Warsaw, 02-004 Warsaw, Poland; [email protected] 2 Department of Gynecology and Gynecological Oncology, Military Institute of Medicine, 04-349 Warsaw, Poland; [email protected] * Correspondence: [email protected] Abstract: The pathogenesis of many serious diseases, including cancer, is closely related to distur- bances in the angiogenesis process. Angiogenesis is essential for the progression of tumor growth and metastasis. The tumor microenvironment (TME) has immunosuppressive properties, which con- tribute to tumor expansion and angiogenesis. Similarly, the uterine microenvironment (UME) exerts a tolerogenic (immunosuppressive) and proangiogenic effect on its cells, promoting implantation and development of the embryo and placenta. In the TME and UME natural killer (NK) cells, which otherwise are capable of killing target cells autonomously, enter a state of reduced cytotoxicity or anergy. Both TME and UME are rich with factors (e.g., TGF-β, glycodelin, hypoxia), which support a conversion of NK cells to the low/non-cytotoxic, proangiogenic CD56brightCD16low phenotype. It is plausible that the phenomenon of acquiring proangiogenic and low cytotoxic features by NK cells is not only limited to cancer but is a common feature of different angiogenesis-dependent diseases (ADDs). In this review, we will discuss the role of NK cells in angiogenesis disturbances associated Citation: Radomska-Le´sniewska, D.M.; with cancer and other selected ADDs. Expanding the knowledge of the mechanisms responsible Białoszewska, A.; Kami´nski,P. -

Identification of Key Mirna‑Mrna Pairs in Septic Mice by Bioinformatics Analysis
3858 MOLECULAR MEDICINE REPORTS 20: 3858-3866, 2019 Identification of key miRNA‑mRNA pairs in septic mice by bioinformatics analysis JIANXIN CHEN1,2, MIN LIN3 and SEN ZHANG1 1Department of Colorectal Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi 530021; 2The First Department of Gastrointestinal Surgery, The Affiliated Hospital of Putian University; 3School of Information Engineering, Putian University, Putian, Fujian 351100, P.R. China Received April 11, 2019; Accepted July 26, 2019 DOI: 10.3892/mmr.2019.10594 Abstract. Sepsis is one of the most common causes of death Introduction among critically ill patients in intensive care units worldwide; however, the microRNAs (miRNAs/miRs) involved in the Sepsis is defined as a life‑threatening organ dysfunction caused sepsis process (and their target genes) are largely unknown. by a dysregulated host response to an infection (1). Sepsis is The present study integrated miRNA and mRNA datasets particularly lethal as it often follows a linear continuum from to elucidate key sepsis-related miRNA‑mRNA pairs. The systemic inflammatory response syndrome through to septic datasets, GSE74952 and GSE55238 were downloaded from shock and organ function failure (2-4). Despite advancements the Gene Expression Omnibus. By performing bioinformatics in antibiotic therapy, immunotherapy and resuscitative strate- analysis such as GEO2R, miRNA target gene prediction, Gene gies, sepsis remains the leading cause of death in intensive Ontology analysis, Kyoto Encyclopedia of Genes and Genomes care units (5). pathway analysis and miRNA‑mRNA network analysis, a total In the pathophysiological process of sepsis, certain gene of four sepsis-related miRNA‑mRNA pairs were successfully expression levels markedly change in vivo and contribute to obtained. -

Mouse CD Marker Chart Bdbiosciences.Com/Cdmarkers
BD Mouse CD Marker Chart bdbiosciences.com/cdmarkers 23-12400-01 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD1d CD1.1, CD1.2, Ly-38 Lipid, Glycolipid Ag + + + + + + + + CD104 Integrin b4 Laminin, Plectin + DNAX accessory molecule 1 (DNAM-1), Platelet and T cell CD226 activation antigen 1 (PTA-1), T lineage-specific activation antigen 1 CD112, CD155, LFA-1 + + + + + – + – – CD2 LFA-2, Ly-37, Ly37 CD48, CD58, CD59, CD15 + + + + + CD105 Endoglin TGF-b + + antigen (TLiSA1) Mucin 1 (MUC1, MUC-1), DF3 antigen, H23 antigen, PUM, PEM, CD227 CD54, CD169, Selectins; Grb2, β-Catenin, GSK-3β CD3g CD3g, CD3 g chain, T3g TCR complex + CD106 VCAM-1 VLA-4 + + EMA, Tumor-associated mucin, Episialin + + + + + + Melanotransferrin (MT, MTF1), p97 Melanoma antigen CD3d CD3d, CD3 d chain, T3d TCR complex + CD107a LAMP-1 Collagen, Laminin, Fibronectin + + + CD228 Iron, Plasminogen, pro-UPA (p97, MAP97), Mfi2, gp95 + + CD3e CD3e, CD3 e chain, CD3, T3e TCR complex + + CD107b LAMP-2, LGP-96, LAMP-B + + Lymphocyte antigen 9 (Ly9),