Cooling Characterization and Practical Utilization of Sub-Micron Slurry Ice for the Chilling of Fresh Seafood
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The BG News April 15, 1999
Bowling Green State University ScholarWorks@BGSU BG News (Student Newspaper) University Publications 4-15-1999 The BG News April 15, 1999 Bowling Green State University Follow this and additional works at: https://scholarworks.bgsu.edu/bg-news Recommended Citation Bowling Green State University, "The BG News April 15, 1999" (1999). BG News (Student Newspaper). 6484. https://scholarworks.bgsu.edu/bg-news/6484 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 License. This Article is brought to you for free and open access by the University Publications at ScholarWorks@BGSU. It has been accepted for inclusion in BG News (Student Newspaper) by an authorized administrator of ScholarWorks@BGSU. ■" * he BG News Women rally to 'take back the night' sexual assault. istration vivors, they will be able to relate her family. By WENDY SUTO Celesta Haras/ti, a resident of buildings, rain to parts of her story, Kissinger "The more I tell my story, the The BG News BG and a W4W member, said the or shine. The said. less shame and guilt I feel," Kissinger said. "For my situa- Women (and some men) will rally is about issues that are con- keynote "When I decided to disclose tion, I'm glad I didn't tell my take to the streets tonight, pro- sidered taboo by society, such as speaker, my sexual abuse to my family, I parents right away because I claiming a public statement in an rape and incest. She has attended Kendel came out of the closet complete- think it would have been a worse attempt to "Take Back the Night" several TBTN marches at the Kissinger, a ly," Kissinger said. -
![[Japan] SALA GIOCHI ARCADE 1000 Miglia](https://docslib.b-cdn.net/cover/3367/japan-sala-giochi-arcade-1000-miglia-393367.webp)
[Japan] SALA GIOCHI ARCADE 1000 Miglia
SCHEDA NEW PLATINUM PI4 EDITION La seguente lista elenca la maggior parte dei titoli emulati dalla scheda NEW PLATINUM Pi4 (20.000). - I giochi per computer (Amiga, Commodore, Pc, etc) richiedono una tastiera per computer e talvolta un mouse USB da collegare alla console (in quanto tali sistemi funzionavano con mouse e tastiera). - I giochi che richiedono spinner (es. Arkanoid), volanti (giochi di corse), pistole (es. Duck Hunt) potrebbero non essere controllabili con joystick, ma richiedono periferiche ad hoc, al momento non configurabili. - I giochi che richiedono controller analogici (Playstation, Nintendo 64, etc etc) potrebbero non essere controllabili con plance a levetta singola, ma richiedono, appunto, un joypad con analogici (venduto separatamente). - Questo elenco è relativo alla scheda NEW PLATINUM EDITION basata su Raspberry Pi4. - Gli emulatori di sistemi 3D (Playstation, Nintendo64, Dreamcast) e PC (Amiga, Commodore) sono presenti SOLO nella NEW PLATINUM Pi4 e non sulle versioni Pi3 Plus e Gold. - Gli emulatori Atomiswave, Sega Naomi (Virtua Tennis, Virtua Striker, etc.) sono presenti SOLO nelle schede Pi4. - La versione PLUS Pi3B+ emula solo 550 titoli ARCADE, generati casualmente al momento dell'acquisto e non modificabile. Ultimo aggiornamento 2 Settembre 2020 NOME GIOCO EMULATORE 005 SALA GIOCHI ARCADE 1 On 1 Government [Japan] SALA GIOCHI ARCADE 1000 Miglia: Great 1000 Miles Rally SALA GIOCHI ARCADE 10-Yard Fight SALA GIOCHI ARCADE 18 Holes Pro Golf SALA GIOCHI ARCADE 1941: Counter Attack SALA GIOCHI ARCADE 1942 SALA GIOCHI ARCADE 1943 Kai: Midway Kaisen SALA GIOCHI ARCADE 1943: The Battle of Midway [Europe] SALA GIOCHI ARCADE 1944 : The Loop Master [USA] SALA GIOCHI ARCADE 1945k III SALA GIOCHI ARCADE 19XX : The War Against Destiny [USA] SALA GIOCHI ARCADE 2 On 2 Open Ice Challenge SALA GIOCHI ARCADE 4-D Warriors SALA GIOCHI ARCADE 64th. -
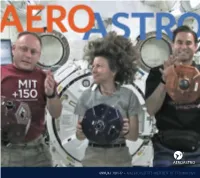
Aeroastro-2011-12.Pdf
ANNUAL 2011-12 • MASSACHUSETTS INSTITUTE OF TECHNOLOGY Editors Department Head Associate Head Editor & Director of Communications Jaime Peraire Karen Willcox William T.G. Litant [email protected] [email protected] [email protected] AeroAstro is published annually by the Massachusetts Institute of Technology Department of Aeronautics and Astronautics, 33-240, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA. http://www.mit.aero AeroAstro No. 9, July 2012 ©2012 The Massachusetts Institute of Technology. All rights reserved. Except where noted, photographs by William Litant/MIT DESIGN Opus Design www.opusdesign.us Cover: Alumni astronauts (from left) Mike Fincke (AeroAstro, BSc ’89), Cady Coleman (ChemE, BSc ’83), and Greg Chamitoff (AeroAstro, PhD ’82), wearing MIT 150 anniversary t-shirts, send video greetings from the International Space Station to high school students around the world participating in AeroAstro’s 2011 Zero Robotics competition. Each is accom- panied by a Space System’s Lab SPHERE microsatellite. For more on Zero Robotics and SPHERES, turn to page 25 (Video grab via NASA). Cert no. XXX-XXX-000 THESE ARE EXCITING TIMES FOR AEROASTRO. Our research funding has risen by 40 percent over the past three years: there are more than 170 research projects in our labs and centers repre- senting $28 million in expenditures by the Department of Defense, NASA, other federal agencies and departments, and the aerospace industry. Our incoming sophomore class size is up by 48 percent over last year. Our faculty now includes a former secretary to the Air Force, a former astronaut, a former NASA associate administrator, a former USAF chief scientist, nine National Academy of Engineering members, nine Amer- ican Institute of Aeronautics and Astronautics Fellows, two Guggenheim Indeed, we have a number of great projects and initiatives ramping Medal recipients, and two AIAA Reed Aeronautics Award recipients. -

THE CINCINNATI MYTH by Eliana Johnson Saturday Night Massacre, and IRS Discrimination Wasn’T Just the Work of Rogue Agents
2013_07_01 subscribe:cover61404-postal.qxd 6/11/2013 8:18 PM Page 1 July 1, 2013 $4.99 JAY NORDLINGER: The Left’s Racial Putdowns KEVIN D. WILLIAMSON ON RAND AND STEINBECK JOHN J. MILLER HENRY OLSEN: IS RAND PAUL THE ANSWER? ON HARRY JAFFA Daniiel Foster w eliiaannaa Johnson w roobb lloonngg $4.99 tthhee eeDDittoorss 26 0 74820 08155 6 www.nationalreview.com base:milliken-mar 22.qxd 6/10/2013 2:27 PM Page 1 TOC:QXP-1127940144.qxp 6/12/2013 2:33 PM Page 1 Contents JULY 1, 2013 | VOLUME LXV, NO. 12 | www.nationalreview.com ON THE COVER Page 16 The Cincinnati Myth John J. Miller on Harry V. Jaffa p. 32 Two Cincinnati employees have told the House Oversight Committee that BOOKS, ARTS they were taking orders from Washington. One of those employees told & MANNERS the committee he began singling out tea- 41 LINCOLN’S PATH, STILL Jay Winik reviews Lincoln party applications at the request of a Unbound: How an Ambitious supervisor who told him “Washington, Young Railsplitter Saved the American Dream—And D.C., wanted some cases.” Eliana Johnson How We Can Do It Again, by Rich Lowry. COVER: ROMAN GENN 42 GREATNESS IN A DARK TIME ARTICLES Charles J. Cooper reviews Saving Justice: Watergate, the 16 THE CINCINNATI MYTH by Eliana Johnson Saturday Night Massacre, and IRS discrimination wasn’t just the work of rogue agents. Other Adventures of a Solicitor General, by Robert H. Bork. 18 ONE-PARTY TAXMEN by Daniel Foster Is it time to un-reform the civil service? 45 EPIC OF A NATION Daniel Johnson reviews Flight of 21 BUREAUCRATIC ROT by Rob Long the Eagle: The Grand Strategies A government, like a fish, decays from its guts. -

IMPROVE Nephelometer Sops.Pdf
QUALITY ASSURANCE/QUALITY CONTROL DOCUMENTATION SERIES TITLE SITE SELECTION FOR OPTICAL MONITORING EQUIPMENT (IMPROVE PROTOCOL) TYPE STANDARD OPERATING PROCEDURE NUMBER 4050 DATE FEBRUARY 1994 AUTHORIZATIONS TITLE NAME SIGNATURE ORIGINATOR Ivar J. Rennat PROJECT MANAGER James H. Wagner PROGRAM MANAGER David L. Dietrich QA MANAGER Gloria S. Mercer OTHER REVISION HISTORY REVISION CHANGE DATE AUTHORIZATIONS NO. DESCRIPTION 1.0 Add responsibilities and equipment. October 1996 Number 4050 Revision 1.0 Date OCT 1996 Page i of i TABLE OF CONTENTS Section Page 1.0 PURPOSE AND APPLICABILITY 1 2.0 RESPONSIBILITIES 2 2.1 Program Manager 2 2.2 Project Manager 2 2.3 Field Specialist 2 2.4 Local (On-Site) Contact 3 3.0 REQUIRED EQUIPMENT AND MATERIALS 3 4.0 METHODS 4 4.1 Nephelometer Site Selection Methods 4 4.1.1 Locating Potential Sites 4 4.1.2 Reviewing and Selecting Potential Sites 4 4.1.3 Finalizing Site Selection 4 4.2 Transmissometer Site Selection Methods 5 4.2.1 Siting Criteria 5 4.2.2 Locating Potential Sites 5 4.2.3 Reviewing and Selecting Potential Sites 5 4.2.4 Finalizing Site Selection 5 Number 4050 Revision 1.0 Date OCT 1996 Page 1 of 6 1.0 PURPOSE AND APPLICABILITY This standard operating procedure (SOP) outlines site selection criteria for optical monitoring instruments operated according to IMPROVE Protocol. Documented site selection criteria and procedures assure consistent, quality data at sites that exhibit most or all of the following characteristics: • Be located in an area representative of the air mass to be monitored • Be -

Optimal Storage Conditions for Fresh Farmed Tilapia (Oreochromis Niloticus) Fillets
Optimal storage conditions for fresh farmed tilapia (Oreochromis niloticus) fillets By: CYPRIAN OGOMBE ODOLI Supervisors: Sigurjón Arason and Emilía Martinsdóttir MS committee: Hannes Magnússon, Hélène L. Lauzon and Kolbrún Sveinsdóttir Thesis submitted in partial fulfilment of the requirements for the Degree of MASTERS IN SCIENCE (60 ECTS units) Department of Food Science and Nutrition University of Iceland May 2009 Optimal storage conditions for fresh farmed tilapia (Oreochromis niloticus) fillets Cyprian Ogombe Odoli Supervisors: Sigurjón Arason and Emilía Martinsdóttir MS committee: Hannes Magnússon, Hélène L. Lauzon and Kolbrún Sveinsdóttir Thesis submitted in partial fulfilment of the requirements for the Degree of MASTERS IN SCIENCE (60 ECTS units) Department of Food Science and Nutrition Faculty of Science University of Iceland May 2009 Declaration I wish to declare that this work has sole been done by me and has never been submitted at any other university before. ___________________ Cyprian Ogombe Odoli Dedication I dedicate this thesis to my daughter Sharon for growing up at early age without seeing her father. More so, my beloved wife Lillian Awuor for accepting a lonesome life while I pursuit academic accomplishment. ii Abstract The main aim was to establish optimal storage conditions for fresh tilapia fillets, by determining its shelf life from the sensory and microbiological evaluation, as well as monitoring its physical-chemical properties. With this intent, Nile tilapia (Oreochromis niloticus) farmed in recirculation aquaculture system was filleted and packaged in 100% air and 50% CO2: 50% N2 MA prior to storage at different temperature; 1˚C and -1˚C. Initial samples from filleting (control d0) were also evaluated for comparison. -

Cold Chain in Seafood Industry
COLD CHAIN IN SEAFOOD INDUSTRY Alex Augusto Gonçalves1, Ph.D and Francisco Blaha2, MSc. Abstract: When seafood is freshly caught eating quality is high, but over time quality will deteriorate and eventually will become unsuitable for consumption. Maintenance of the cold chain and careful handling are a fundamental part in minimizing seafood spoilage. The cold chain in seafood industry is a temperature chain and begins once the seafood is caught. From the quality and regulatory perspective fresh seafood means that has been stored at 0°C and for frozen seafood it means –18°C or colder (from the sea to the consumer). The cold chain is broken every time the temperature of the seafood rises above 1°C. Fluctuations in the cold chain increases quality loss, these losses cannot be reversed by any means after the event. There are many avoidable and unavoidable occurrences that cause fluctuation in the cold chain. Good chilling and/or freezing practices on board harvest vessels are a condition sine qua non for maximizing the best possible quality of all seafoods. Best practices on seafood handling and the minimization of temperature fluctuations are paramount for seafood distributed under refrigeration (i.e., chilled and/or frozen) to maintain its quality and maximize its shelf–life. The present chapter addresses some of the developments in seafood cold chain under four mainly headings: i) cold chain in seafood industry; ii) selected aspects of refrigeration (chilling and freezing) process (including equipment and storage conditions); iii) safety and shelf–life issues in seafood cold chain; iv) regulation and legislation. 1. -
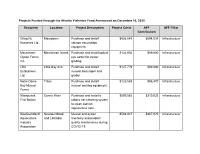
Projects Funded Through the Atlantic Fisheries Fund Announced on December 16, 2020
Projects Funded through the Atlantic Fisheries Fund Announced on December 16, 2020 Recipient Location Project Description Project Costs AFF AFF Pillar Contribution Grieg NL Marystown Purchase and install $926,045 $694,534 Infrastructure Nurseries Ltd. salmon vaccination equipment. Merasheen Merasheen Island Purchase and install optical $124,930 $99,900 Infrastructure Oyster Farms eye sorter for oyster Inc. grading. LBA Little Bay Arm Purchase and install $127,779 $99,900 Infrastructure Enterprises mussel declumper and Ltd. grader. Notre Dame Triton Purchase and install $120,588 $96,470 Infrastructure Bay Mussel mussel socking equipment. Farms Miawpukek Conne River Purchase and install a $899,583 $319,623 Infrastructure First Nation robotic net cleaning system to clean salmon aquaculture nets. Newfoundland Newfoundland Mussel and oyster $583,837 $467,070 Infrastructure Aquaculture and Labrador inventory and product Industry quality maintenance during Association COVID-19. Newfoundland Milltown Purchase of robotic net $1,752,339 $1,261,754 Infrastructure Aqua Service cleaning systems Ltd. for aquaculture pens. Bobby Noble Lewisporte Purchase and install $250,771 $99,882 Infrastructure automated longline system CRS Fishing Grates Cove Purchase and install $136,960 $99,900 Infrastructure Enterprises automated longline system Ltd. Challenger 92 Fogo Island Purchase and install $102,584 $82,067 Infrastructure Ltd. automated longline system Reginald Baine Harbour Purchase and install $9,786 $7,829 Infrastructure Keating automated jiggers and insulated boxes. Jerome Ward South East Bight Purchase and install $4,896 $3,917 Infrastructure automated jiggers and insulated boxes. Matthew Pasadena Purchase and install $21,891 $17,513 Infrastructure Rumbolt automated jiggers. Mervin Hollett Arnold's Cove Purchase and install $17,991 $12,515 Infrastructure automated jiggers and insulated boxes. -

\0-9\0 and X ... \0-9\0 Grad Nord ... \0-9\0013 ... \0-9\007 Car Chase ... \0-9\1 X 1 Kampf ... \0-9\1, 2, 3
... \0-9\0 and X ... \0-9\0 Grad Nord ... \0-9\0013 ... \0-9\007 Car Chase ... \0-9\1 x 1 Kampf ... \0-9\1, 2, 3 ... \0-9\1,000,000 ... \0-9\10 Pin ... \0-9\10... Knockout! ... \0-9\100 Meter Dash ... \0-9\100 Mile Race ... \0-9\100,000 Pyramid, The ... \0-9\1000 Miglia Volume I - 1927-1933 ... \0-9\1000 Miler ... \0-9\1000 Miler v2.0 ... \0-9\1000 Miles ... \0-9\10000 Meters ... \0-9\10-Pin Bowling ... \0-9\10th Frame_001 ... \0-9\10th Frame_002 ... \0-9\1-3-5-7 ... \0-9\14-15 Puzzle, The ... \0-9\15 Pietnastka ... \0-9\15 Solitaire ... \0-9\15-Puzzle, The ... \0-9\17 und 04 ... \0-9\17 und 4 ... \0-9\17+4_001 ... \0-9\17+4_002 ... \0-9\17+4_003 ... \0-9\17+4_004 ... \0-9\1789 ... \0-9\18 Uhren ... \0-9\180 ... \0-9\19 Part One - Boot Camp ... \0-9\1942_001 ... \0-9\1942_002 ... \0-9\1942_003 ... \0-9\1943 - One Year After ... \0-9\1943 - The Battle of Midway ... \0-9\1944 ... \0-9\1948 ... \0-9\1985 ... \0-9\1985 - The Day After ... \0-9\1991 World Cup Knockout, The ... \0-9\1994 - Ten Years After ... \0-9\1st Division Manager ... \0-9\2 Worms War ... \0-9\20 Tons ... \0-9\20.000 Meilen unter dem Meer ... \0-9\2001 ... \0-9\2010 ... \0-9\21 ... \0-9\2112 - The Battle for Planet Earth ... \0-9\221B Baker Street ... \0-9\23 Matches .. -

Design and Fabrication of Slurry Ice Generator
International Journal for Research in Engineering Application & Management (IJREAM) ISSN : 2454-9150 Vol-03, Issue-07, Oct 2017 Design and Fabrication of Slurry Ice Generator Vikas K Tawde Department of Mechanical Engineering, Vidyavardhini’s College of Engineering and Technology, Vasai, Mumbai, India. Abstract: This paper proposes the Design and Fabrication of a slurry ice generator which produces slurry ice, a mixture of water and ice wherein the ice is in the form of spherical crystals of about 0.1 to 1mm in diameter. It provides 3 to 4 times better cooling compared to flaked ice owing to larger heat transfer area. Slurry ice finds application in food preservation especially in the fishing industry. In India, flaked ice is used for seafood preservation. Introduction of slurry ice in the fishing industry will result in better quality of produce. This paper is focused on the design calculations and the selection of proper components wherein the conventional ice scraper is replaced by an Archimedes’ screw to eliminate the use of circulation pump. The refrigeration system works on a simple vapor compression cycle. Ice crystals are formed on the inner surface of shell and coil type evaporator, and, scraped by the Archimedes’ screw. Keywords — Ice Slurry, Archimedes’ screw, Newtonian, pumpability, R134a, isentropic, coefficient of performance. and heat exchangers. Ice slurries can also be pumped through NTRODUCTION I. I veins and lungs to lower temperature much faster. Because of The fishing industry in India makes use of flaked ice for Pumpability slurry ice with nozzles can be used in fire preservation. Often times the quality of product received by extinguishing applications. -

A Revolution in Food Preservation Why Flake Ice Doesn’T Work Anymore…
A REVOLUTION IN FOOD PRESERVATION WHY FLAKE ICE DOESN’T WORK ANYMORE… Modern consumers have become extremely aware of how important food quality is to their nutrition and way of living. Therefore, they continue to demand food that is fresh, of higher quality, and delivered quickly to the market. This puts seafood harvesters, aquaculture producers and processors in a position where they need to adapt, but don’t have the right means to preserve harvest for long periods of time. Although it is the cheapest option, flake ice cannot do the job anymore as it: • damages the product surface and causes breakage • leads to shrinkage and weight loss The increasing demand • and creates an environment for bacteria to cause for better and fresher food quicker product spoilage. puts an even stronger pressure on aquaculture All that decreases the product value and minimizes the producers and food profit margins aquaculture manufacturers and food processors. processors need to scale in the long run. …AND SLURRY ICE DOESN’T SOLVE THE PROBLEM EITHER Liquid ice solutions, like slurry ice, use small ice crystals to absorb heat from fresh products. Usually, they have good flow properties and are easily distributed. As slurry ice creates a uniform contact with the product surface, it’s often considered an improvement over flake ice. However, this is only partial. Slurry ice still lacks the deepest level of cooling transfer to products, which may lead to spoilage. Sometimes, this results in seafood harvesters, aquaculture producers and processors to use additives in the preservation process, which is the opposite of the consumer demand for more organic, preservatives-free products. -

Genetic Testing and the Future of Cerebral Palsy Malpractice Cases
What’s the VERDICT? Genetic testing and the future of cerebral palsy malpractice cases Would genetic testing have prevented this lawsuit? Joseph S. Sanfilippo, MD, MBA, and Steven R. Smith, MS, JD CASE Mixed CP diagnosed at age 6 months After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initi- ated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks. Her past medical history was largely non- contributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a his- IN THIS tory of asthma, but had had no acute episodes ARTICLE for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial Types of cerebral thyroid studies were abnormal (thyroid-stimulat- palsy ing hormone levels, 7.1 mIU/L), in part due to page 41 lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP Legal monitoring during her last trimester. considerations page 42 Dr. Sanfilippo is Professor, Department Avoiding of Obstetrics, Gynecology, and Reproductive Sciences, University of malpractice claims Pittsburgh, and Director, Reproductive page 46 Endocrinology and Infertility, at Magee-Womens Hospital, Pittsburgh, Pennsylvania. He also serves on the OBG MANAGEMENT Board of Editors. Mr. Smith is Professor Emeritus and Dean Emeritus at California Western School of Law, San Diego, California.