Cold Chain in Seafood Industry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Achieving Immunization Targets with the Comprehensive Effective Vaccine Management (EVM) Framework
WHO/UNICEF JOINT STATEMENT Achieving immunization targets with the comprehensive effective vaccine management (EVM) framework WHO and UNICEF joint statement on the comprehensive EVM framework and the vital role of immunization supply chains in achieving targets set by the Global Vaccine Action Plan (GVAP). The World Health Organization (WHO) and the United can more effectively catalyse immunization supply chain Nations Children’s Fund (UNICEF) are intensifying and improvements in countries. coordinating efforts to catalyse immunization supply chain To that end, this joint statement: improvements and performance in countries through the comprehensive EVM framework – a four-step strategy for 1. Reiterates the vital role of immunization supply chains in continuous immunization supply chain improvement, quality achieving vaccination coverage and equity targets set by management, optimization and innovation. the GVAP; 2. Summarizes the evidence indicating that vaccines are The comprehensive EVM framework builds on the success of not always available when needed; that weak cold chain the EVM initiative launched in 2010 to help countries evaluate systems are putting vaccines at risk and delaying new the performance of their immunization supply chains, and vaccine introduction; and that vaccines are wasted as a benchmark this performance against best-practice standards. result of poor supply chain management; In five years, the EVM initiative has helped to uncover 3. Describes the comprehensive EVM framework and how important shortcomings in the -

Guidance on Developing a National Deployment and Vaccination Plan
Guidance on developing a national deployment and vaccination plan for COVID-19 vaccines INTERIM GUIDANCE 16 NOVEMBER 2020 Guidance on developing a national deployment and vaccination plan for COVID-19 vaccines INTERIM GUIDANCE 16 NOVEMBER 2020 WHO/2019-nCoV/Vaccine_deployment/2020.1 © World Health Organization 2020 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. (http://www.wipo.int/amc/en/mediation/rules/) Suggested citation. Guidance on developing a national deployment and vaccination plan for COVID-19 vaccines. Geneva: World Health Organization; 2020 (WHO/2019-nCoV/Vaccine_deployment/2020.1). -

Chain of Cold Food
Journal of Microbiology & Experimentation Opinion Open Access Chain of cold food Volume 4 Issue 3 - 2017 Opinion Carla Pagano The cold chain is the system formed by each of the steps that Engineer in food, Argentina constitute the process of refrigeration or freezing necessary for the perishable or frozen food to reach the consumer safely. It includes a Correspondence: Carla Pagano, Engineer in food, Avenida whole set of elements and activities necessary to guarantee the quality Escalada 1817, Buenos Aires, Argentina, Tel 0111561270830, Email and safety of a food, from its origin to its consumption. It is called “string” because it is composed of different stages or links. If any Received: March 16, 2017 | Published: March 23, 2017 of the points in the cold chain were compromised, all of it would be affected, damaging the quality and safety of the product. On the one hand it facilitates the microbial development of both altering microorganisms and disease-causing pathogens, and the alteration of the food by degrading enzymatic reactions. Cold on microorganisms When the temperature is reduced, so does the rate of development of the vast majority of microorganisms, preventing a population increase (a group of microorganisms, the psychrophilic, which develop at low temperatures). The cold acts on the metabolism of microorganisms slowing (cooling) to stop (freezing), but does not eliminate them (although it can be seen some microbial mortality, the cold is not hygienizing, as it is the intense heat). Between -4ºC and -7ºC the growth of pathogenic microorganisms during transport, which takes place between the different stages: at the is inhibited. -

THE CINCINNATI MYTH by Eliana Johnson Saturday Night Massacre, and IRS Discrimination Wasn’T Just the Work of Rogue Agents
2013_07_01 subscribe:cover61404-postal.qxd 6/11/2013 8:18 PM Page 1 July 1, 2013 $4.99 JAY NORDLINGER: The Left’s Racial Putdowns KEVIN D. WILLIAMSON ON RAND AND STEINBECK JOHN J. MILLER HENRY OLSEN: IS RAND PAUL THE ANSWER? ON HARRY JAFFA Daniiel Foster w eliiaannaa Johnson w roobb lloonngg $4.99 tthhee eeDDittoorss 26 0 74820 08155 6 www.nationalreview.com base:milliken-mar 22.qxd 6/10/2013 2:27 PM Page 1 TOC:QXP-1127940144.qxp 6/12/2013 2:33 PM Page 1 Contents JULY 1, 2013 | VOLUME LXV, NO. 12 | www.nationalreview.com ON THE COVER Page 16 The Cincinnati Myth John J. Miller on Harry V. Jaffa p. 32 Two Cincinnati employees have told the House Oversight Committee that BOOKS, ARTS they were taking orders from Washington. One of those employees told & MANNERS the committee he began singling out tea- 41 LINCOLN’S PATH, STILL Jay Winik reviews Lincoln party applications at the request of a Unbound: How an Ambitious supervisor who told him “Washington, Young Railsplitter Saved the American Dream—And D.C., wanted some cases.” Eliana Johnson How We Can Do It Again, by Rich Lowry. COVER: ROMAN GENN 42 GREATNESS IN A DARK TIME ARTICLES Charles J. Cooper reviews Saving Justice: Watergate, the 16 THE CINCINNATI MYTH by Eliana Johnson Saturday Night Massacre, and IRS discrimination wasn’t just the work of rogue agents. Other Adventures of a Solicitor General, by Robert H. Bork. 18 ONE-PARTY TAXMEN by Daniel Foster Is it time to un-reform the civil service? 45 EPIC OF A NATION Daniel Johnson reviews Flight of 21 BUREAUCRATIC ROT by Rob Long the Eagle: The Grand Strategies A government, like a fish, decays from its guts. -

Cold Chain 101 Cold Chain 101
Cold Chain 101 Cold Chain 101 Even some railroads are providing temperature- “Logistics providers are aware of the controlled shipping, with the ability to plug in reefers. increasing need for controlling temperatures in multiple ranges, and are Temperature Monitoring building out their facilities accordingly.” Even with these advances, temperature controlled products still need to be monitored. The reason is What is Cold Chain? simple. Equipment, no matter how new, sometimes Whether a product is sitting in a warehouse or being fails. Accidents happen. shipped across the country or across the globe, if temperature matters, that product is part of the cold Human error also is a factor. New logistics personnel, chain. Until recently, the term “cold chain” referred impressed with the thermal sensitivity of vaccines and only to products that needed to be cooled. Now it has the potentially deadly repercussions of temperature expanded to also include products that must be kept at excursions, have placed packages in coolers until room temperature. More recently, as products that must refrigerated carriers arrived for pickup. Some of those be protected from the cold enter the mix, “cold chain” is shipments actually froze, causing the very damage morphing to “controlled temperature.” handlers tried to prevent. Other times, truckers actually disconnected cargo refrigeration equipment to conserve fuel. Seafood and pharmaceuticals are two obvious examples of products needing cold chain handling, but the type of During transshipment, when cargo containers are products requiring controlled temperatures is expanding offloaded from ships and transferred to other vessels, dramatically. Chemical products (like paints and coatings), reefers may not be plugged in to shore power sometimes use temperature controlled shipping to prevent Likewise, once loaded, they may not be plugged back them from separating so they will maintain consistency and, into the ship’s power. -

Impacts of Vaccine Cold Chain Logistics on Vaccine Epidemiology
IMPACTS OF VACCINE COLD CHAIN LOGISTICS ON VACCINE EPIDEMIOLOGY by Tina-Marie Assi BS, McGill University, 2007 MPH, University of Pittsburgh, 2009 Submitted to the Graduate Faculty of Graduate School of Public Health in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Pittsburgh 2011 UNIVERSITY OF PITTSBURGH GRADUATE SCHOOL OF PUBLIC HEALTH This dissertation was presented by Tina-Marie Assi It was defended on June 30, 2011 and approved by Shawn T. Brown, PhD Assistant Professor Department of Biostatistics Graduate School of Public Health University of Pittsburgh Bryan A. Norman, PhD Associate Professor Department of Industrial Engineering Swanson School of Engineering University of Pittsburgh Jayant Rajgopal, PhD Associate Professor Department of Industrial Engineering Swanson School of Engineering University of Pittsburgh Stephen R. Wisniewski, PhD Professor Department of Epidemiology, Graduate School of Public Health University of Pittsburgh Dissertation Advisor: Bruce Y. Lee, MD, MBA Assistant Professor Departments of Medicine, Epidemiology and Biomedical Informatics School of Medicine and Graduate School of Public Health University of Pittsburgh ii Copyright © by Tina-Marie Assi 2011 iii Bruce Y. Lee, MD, MBA IMPACTS OF VACCINE COLD CHAIN LOGISTICS ON VACCINE EPIDEMIOLOGY Tina-Marie Assi, PhD University of Pittsburgh, 2011 The performance of vaccine logistics systems (i.e., the steps in a supply chain necessary to get vaccines from manufacturers to patients) can impact whether vaccines are delivered at the right time, place and in the right condition for patients during immunization sessions. Immunization coverage in a population depends on a well-functioning vaccine supply chain. If target populations are not immunized before exposure, they are left unprotected against vaccine preventable diseases (VPD’s) and can contribute to infectious disease transmission in their communities. -

Vaccines: Harnessing Science to Drive Innovation for Patients
VACCINES: HARNESSING SCIENCE TO DRIVE INNOVATION FOR PATIENTS BOLDLY INTRODUCTION Advances in science and technology are driving increases in survival and improved quality of life for people around the world. Vaccines represent some of the most impactful public health advances, helping to prevent the spread of many infectious diseases and, in many parts of the world, eliminating some of the most devastating conditions. Examples of vaccine successes are numerous and significant: • Smallpox, at one point one of the deadliest diseases in existence, has been eradicated around the world as a result of vaccination.1 • Following the introduction of the first polio vaccine in 1955, the crippling infectious disease has been eliminated in the United States (U.S.) and by 2015 just 74 cases of the disease were reported around the world.2 3 • The recent introduction of the human papilloma virus (HPV) vaccine has changed the trajectory of cervical cancer, by preventing infection of the HPV strains most likely to cause cancers.4 • A new wave of therapeutic vaccines has the potential to treat diseases, including many cancers.5 As our understanding of the science grows, uncovering the complex biological drivers of many diseases, and regulatory requirements expand, the research and development (R&D) process to develop medicines also grows more challenging. Innovative biopharmaceutical companies are working with stakeholders from across the research and development ecosystem to develop new ways of preventing and treating illnesses, with vaccines at the forefront. As of October 2017, there are more than 260 vaccines in development by biopharmaceutical companies for the treatment or prevention of disease. -

A Study of Waste in the Cold Food Chain and Opportunities for Improvements
A study of waste in the cold food chain and opportunities for improvement Prepared for: Department of Agriculture, Water and the Environment and Refrigerants Australia Phone Web +61 3 9592 9111 www.expertgroup.com.au Table of Contents 1 Executive summary ........................................................................................................... 5 2 Introduction ...................................................................................................................... 6 3 Scope of this study ............................................................................................................. 7 3.1 Refrigerated cold food chain .............................................................................................................................. 8 4 Food sector profiles ........................................................................................................... 9 4.1 Fruit and vegetable sector ............................................................................................................................. 9 4.1.1 Supply chain profile ...................................................................................................................................... 9 4.1.2 Role of the cold food chain ......................................................................................................................... 9 4.2 Meat sector .................................................................................................................................................... -

Unprecedented Famine Risk Threatens 2.5 Million Children
Quarterly Activity Report May 15, 2017 Unprecedented Famine Risk Threatens 2.5 Million Children Amina, with her daughter, holds up her empty cooking pot inside their makeshift home near the town of Ainabo, Somalia. © UNICEF/UN056039/HOLT ime is running out for at least 2.5 million children who million people at risk in the hardest hit regions, the magnitude are at imminent risk of death this year from severe acute and urgency of the situation is overwhelming. By accelerating T malnutrition in 13 countries spanning Africa and the funds through its Bridge Fund, UNICEF USA is doing everything Middle East. Conflict and displacement in these countries, in its power to save children’s lives. As Leila Pakkala, UNICEF exacerbated by drought and water scarcity, have resulted in what Regional Director for Eastern and Southern Africa, said during the United Nations is saying is the largest humanitarian crisis a recent visit to the UNICEF USA offices, “In a time of famine, since the Second World War. Famine has been declared in South impact does not come gradually. It is sudden. If we respond right Sudan and could ensue in Nigeria, Somalia and Yemen. With 80 now, for every dollar received, we’re able to save a child’s life.” Sustained conflict across multiple countries, Unprecedented Humanitarian Crisis which has forced millions of children and families from their homes, has caused Looms Across 13 Countries massive social and economic disruption. It has also resulted in grave violations of children’s rights. In Northeast Nigeria, children who have fled Boko Haram’s attempts to conscript them as child soldiers or kidnap them as child “brides” are now at risk of starvation. -

Optimal Storage Conditions for Fresh Farmed Tilapia (Oreochromis Niloticus) Fillets
Optimal storage conditions for fresh farmed tilapia (Oreochromis niloticus) fillets By: CYPRIAN OGOMBE ODOLI Supervisors: Sigurjón Arason and Emilía Martinsdóttir MS committee: Hannes Magnússon, Hélène L. Lauzon and Kolbrún Sveinsdóttir Thesis submitted in partial fulfilment of the requirements for the Degree of MASTERS IN SCIENCE (60 ECTS units) Department of Food Science and Nutrition University of Iceland May 2009 Optimal storage conditions for fresh farmed tilapia (Oreochromis niloticus) fillets Cyprian Ogombe Odoli Supervisors: Sigurjón Arason and Emilía Martinsdóttir MS committee: Hannes Magnússon, Hélène L. Lauzon and Kolbrún Sveinsdóttir Thesis submitted in partial fulfilment of the requirements for the Degree of MASTERS IN SCIENCE (60 ECTS units) Department of Food Science and Nutrition Faculty of Science University of Iceland May 2009 Declaration I wish to declare that this work has sole been done by me and has never been submitted at any other university before. ___________________ Cyprian Ogombe Odoli Dedication I dedicate this thesis to my daughter Sharon for growing up at early age without seeing her father. More so, my beloved wife Lillian Awuor for accepting a lonesome life while I pursuit academic accomplishment. ii Abstract The main aim was to establish optimal storage conditions for fresh tilapia fillets, by determining its shelf life from the sensory and microbiological evaluation, as well as monitoring its physical-chemical properties. With this intent, Nile tilapia (Oreochromis niloticus) farmed in recirculation aquaculture system was filleted and packaged in 100% air and 50% CO2: 50% N2 MA prior to storage at different temperature; 1˚C and -1˚C. Initial samples from filleting (control d0) were also evaluated for comparison. -

(Meningitis a Vaccine) in a Controlled Temperature Chain (CTC) During Campaigns
WHO/IVB/13.04 Use of MenAfriVac™ (meningitis A vaccine) in a controlled temperature chain (CTC) during campaigns Guidance for immunization programme decision-makers and managers Immunization, Vaccines and Biologicals WHO/IVB/13.04 Use of MenAfriVac™ (meningitis A vaccine) in a controlled temperature chain (CTC) during campaigns Guidance for immunization programme decision-makers and managers Immunization, Vaccines and Biologicals The Department of Immunization, Vaccines and Biologicals thanks the donors whose unspecified financial support has made the production of this document possible. This document was produced by the Expanded Programme on Immunization (EPI) of the Department of Immunization, Vaccines and Biologicals This document forms part 1 of 3 parts of the training guide for the Use of MenAfriVac™ (meningitis A vaccine) in a controlled temperature chain (CTC) during campaigns Ordering code: WHO/IVB/13.04 Printed: July 2013 This publication is available on the Internet at: www.who.int/vaccines-documents/ Copies of this document as well as additional materials on immunization, vaccines and biologicals may be requested from: World Health Organization Department of Immunization, Vaccines and Biologicals CH-1211 Geneva 27, Switzerland • Fax: + 41 22 791 4227 • Email: [email protected] • © World Health Organization 2013 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 3264; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: [email protected]). -
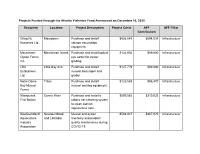
Projects Funded Through the Atlantic Fisheries Fund Announced on December 16, 2020
Projects Funded through the Atlantic Fisheries Fund Announced on December 16, 2020 Recipient Location Project Description Project Costs AFF AFF Pillar Contribution Grieg NL Marystown Purchase and install $926,045 $694,534 Infrastructure Nurseries Ltd. salmon vaccination equipment. Merasheen Merasheen Island Purchase and install optical $124,930 $99,900 Infrastructure Oyster Farms eye sorter for oyster Inc. grading. LBA Little Bay Arm Purchase and install $127,779 $99,900 Infrastructure Enterprises mussel declumper and Ltd. grader. Notre Dame Triton Purchase and install $120,588 $96,470 Infrastructure Bay Mussel mussel socking equipment. Farms Miawpukek Conne River Purchase and install a $899,583 $319,623 Infrastructure First Nation robotic net cleaning system to clean salmon aquaculture nets. Newfoundland Newfoundland Mussel and oyster $583,837 $467,070 Infrastructure Aquaculture and Labrador inventory and product Industry quality maintenance during Association COVID-19. Newfoundland Milltown Purchase of robotic net $1,752,339 $1,261,754 Infrastructure Aqua Service cleaning systems Ltd. for aquaculture pens. Bobby Noble Lewisporte Purchase and install $250,771 $99,882 Infrastructure automated longline system CRS Fishing Grates Cove Purchase and install $136,960 $99,900 Infrastructure Enterprises automated longline system Ltd. Challenger 92 Fogo Island Purchase and install $102,584 $82,067 Infrastructure Ltd. automated longline system Reginald Baine Harbour Purchase and install $9,786 $7,829 Infrastructure Keating automated jiggers and insulated boxes. Jerome Ward South East Bight Purchase and install $4,896 $3,917 Infrastructure automated jiggers and insulated boxes. Matthew Pasadena Purchase and install $21,891 $17,513 Infrastructure Rumbolt automated jiggers. Mervin Hollett Arnold's Cove Purchase and install $17,991 $12,515 Infrastructure automated jiggers and insulated boxes.