Vaccine Storage and Handling Toolkit-March 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Achieving Immunization Targets with the Comprehensive Effective Vaccine Management (EVM) Framework
WHO/UNICEF JOINT STATEMENT Achieving immunization targets with the comprehensive effective vaccine management (EVM) framework WHO and UNICEF joint statement on the comprehensive EVM framework and the vital role of immunization supply chains in achieving targets set by the Global Vaccine Action Plan (GVAP). The World Health Organization (WHO) and the United can more effectively catalyse immunization supply chain Nations Children’s Fund (UNICEF) are intensifying and improvements in countries. coordinating efforts to catalyse immunization supply chain To that end, this joint statement: improvements and performance in countries through the comprehensive EVM framework – a four-step strategy for 1. Reiterates the vital role of immunization supply chains in continuous immunization supply chain improvement, quality achieving vaccination coverage and equity targets set by management, optimization and innovation. the GVAP; 2. Summarizes the evidence indicating that vaccines are The comprehensive EVM framework builds on the success of not always available when needed; that weak cold chain the EVM initiative launched in 2010 to help countries evaluate systems are putting vaccines at risk and delaying new the performance of their immunization supply chains, and vaccine introduction; and that vaccines are wasted as a benchmark this performance against best-practice standards. result of poor supply chain management; In five years, the EVM initiative has helped to uncover 3. Describes the comprehensive EVM framework and how important shortcomings in the -

Case Flight AF- 447 Air France
Work 41 (2012) 222-224 222 DOI: 10.3233/WOR-2012-0160-222 IOS Press Automation under suspicion – case flight AF- 447 Air France Edgard Martins1 and Marcelo Soares2 1Universidade Federal de Pernambuco- Depto de Design- CAA – Caruaru- Pernambuco- Brasil, [email protected] 2UFPE- Universidade Federal de Pernambuco- Depto de Design- CAA – Recife- Pernambuco- Brasil Abstract. The probes allow the pilot to control the aircraft speed was essential to the balance of the flight. Opinions of experts who claim that "the design of the plane would have exercised a not inconsiderable role in the occurrence of a disaster. " These messages revealed a series of important operating errors in a zone of turbulence, "making the plane uncontrollable, leading to a rapid depressurization device, according to these reports. A lawsuit in Toulouse and in Brazil aims to recognition of the liabil- ity of Air France and Airbus not insignificant role in the design and operation of the aircraft in the event of catastrophe. Opin- ions are taken from senior pilots that no commercial aviation training for certain situations abnormal flight that, if realized, could have influenced the pilots of the AF-447 to remove the plane's fatal dive show what experiments performed in simulators for military pilots, who are permanently subject to critical flight situations. Keywords: processing information; human error; trainning 1. Introduction into the Atlantic 200 aircraft and crashed into the Atlantic like saw at figure 1.* The Air France flight from Rio de Janeiro to Paris that crashed in 2009 plummeted 38,000 ft in just three minutes and 30 seconds because pilots lost vital speed data, France’s Bureau of Investigation and Analysis (BEA) said Friday. -

6-Speed Blender
LIMITED WARRANTY* ONE (1) YEAR: Your small kitchen appliance is warranted to the original purchaser to be free from any manufacturing defects under normal use and conditions for one (1) year, cord excluded. During that period, should the appliance fail to operate properly, return the appliance with your sales receipt to the store where purchased. If you use your appliance for household use and according to instructions, it should give you years of satisfactory service. This product warranty covers only the original consumer purchaser of the product. WARRANTY IS ONLY VALID WITH A DATED PROOF OF PURCHASE. To guarantee repair or replace without charge, a dated sales receipt showing purchase within the limited warranty period* must accompany the appliance. Without a sales receipt, warranty will be estimated according to the appliance's manufactured date. A comparable appliance should arrive within 2-3 weeks. 10 Speed Blender However, in case an appliance is not covered by warranty, correspondence offering alternatives will be mailed to you. with 48oz. Glass Jar During the one-year warranty period, a product with a defect will be either repaired or replaced with a reconditioned comparable model (at our option) when the product is returned to our Service Center. (See the “Returns” section below). The repaired or replacement product will be in warranty for the remaining balance of the one-year warranty period and an additional one-month period. This limited warranty covers appliances purchased and used within the 50 contiguous states plus the District of Columbia and does NOT cover: - Damages caused by unreasonable use, neglect, normal wear and tear, commercial use, improper assembly or installation of product. -

March 18-19-20 3 Chamber News
VOL. 31 ISSUE 03 A MONTHLY MEMBER PUBLICATION OF THE HASTINGS AREA CHAMBER OF COMMERCE March 2016 Inside... 2 From the President’s Desk March 18-19-20 3 Chamber News 4 Ribbon Cuttings/Salutes/Events 5 Annual Banquet Sponsors 9 Chamber News/Area Events & News 10 Membership Recognition 2016 11 Banquet Award Winners 12 Chamber Staff/Calendar It’s Whoopers and Hoopers time! We already have 59 teams registered and are still going strong. What we YOUR CHAMBER IS... need now are 95 more volunteers to • A Not for profit 501 C6, private, business organization. help at the ticket tables. Shifts are only • Supported by more than 700 businesses, professional, and industrial firms, community members, organiza- two hours long and when you volunteer you will receive a FREE admission to any tions, and utilities. of the Whoopers & Hoopers Tournament • An investment is based on classification and number games! If you are interested in helping of employees. Investments are 80% tax deductible as an ordinary business expense. support a wonderful event that brings • Working for the business community, and it can work several thousands of dollars to Hastings then for you – it is the business connection! please contact the Hastings Area Chamber of • Always seeking to develop programs that will establish Commerce to see what times and locations the Hastings area as an attractive place to live and do business. are available. Thanks for your support! • A volunteer organization, supported by a professional staff. Chamber Staff For more info on any events Be in the KNOW about the Hastings Chamber please call the Chamber at 461-8400 • Business Before Hours • Business After Hours • Special Events • Discounts & Promotions • And More.. -

Three Minutes
MICHAEL MORTENSON Three Minutes he Conoco gas station was deserted when I rolled up. TSixteen fully-functioning, state-certified gas pumps and no one was interested, poor things. Surely, I couldn’t be the only one traveling along this desert stretch of I-15 with a thirsty car. I glanced across the street to the big, red-and-white num - bers on the Shell sign: $2.69 a gallon. Same exact price as the Conoco. No economic excuse for this empty gas station. I eased the car up to the nearest pump and the brakes let out an inglo - rious screeching, like a cheese grater on a chalkboard. I got out of the car and stretched. Oh, the glory of arching my back and reaching my arms to the sky! Fishing my squashed wallet from my pocket, I inserted a card into the machine. The gas pump gave an obstinate beep and spit the card back out. Its display flickered dimly, then went blank. Maybe it hadn’t read my card right. I re-inserted the card, only for the machine to spit it right back out again like a two-year-old with Gerber peas. I guess there are those that just can’t stomach Wells Fargo. I gave the keypad a couple of jabs, hoping—though not really expecting—that I might prod some sensibility into the 61 T HE R ESTORED G OSPEL machine. Meep . meep . meep . went the machine. No luck. I shoved my wallet back into my pocket. Fine. Be that way. I drove the car around to another pump. -

Guidance on Developing a National Deployment and Vaccination Plan
Guidance on developing a national deployment and vaccination plan for COVID-19 vaccines INTERIM GUIDANCE 16 NOVEMBER 2020 Guidance on developing a national deployment and vaccination plan for COVID-19 vaccines INTERIM GUIDANCE 16 NOVEMBER 2020 WHO/2019-nCoV/Vaccine_deployment/2020.1 © World Health Organization 2020 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. (http://www.wipo.int/amc/en/mediation/rules/) Suggested citation. Guidance on developing a national deployment and vaccination plan for COVID-19 vaccines. Geneva: World Health Organization; 2020 (WHO/2019-nCoV/Vaccine_deployment/2020.1). -

The Kent Rotary Nooz
TheThe KentKent RotaryRotary NoozNooz www.kentrotary.com 2017-18 A Year Without Fear Next Meeting Make Ups Please send your make ups to October 24, 2017 Bill Dugovich [email protected] Ramada Hotel Rotary Cares Catered by Mitzel’s Kitchen Someone need a card? Send info to 22318 84th Ave S. [email protected] Kent, WA 98032 Noon World Polio Day This will be a “Don Gregory Production” and all about our progress with eradicating polio from the earth. It’s a good news story. This is the day in 1945 that the United Nations was born. Andy was born two years later. Scribe: Your Humble Scribe™ Invocation/Inspiration: Becky Hanks At the Last Meeting October 17, 2017 Your Humble Scribe™ was present and taking notes. In a blatant attempt to “earn points,” Billy the Pez rang the bell at EXACTLY 12:30 to begin our meeting. He displayed a photo of a baseball player and asked what happened on this day in 1835. Of course, no one knew. Turns out the Texas Rangers moved from outlaws to law enforcement. Nothing about baseball after all! With that, Harry Williams led us in an “Exciting” Pledge of Allegiance with no one taking a knee (that I could see). Ryan Rehberg was AWOL (despite Billy the Prez having a Disney graphic on the big screen), so Andy Wangstad delivered an inspirational quote: “Nobody makes you angry; You decide to use anger as a response.” Well, isn’t that true. New Member Kay Cook has proposed Coleen Perry for membership with the classification of Commercial Moving and Logistics. -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -

Illinois Rules of the Road 2021 DSD a 112.35 ROR.Qxp Layout 1 5/5/21 9:45 AM Page 1
DSD A 112.32 Cover 2021.qxp_Layout 1 1/6/21 10:58 AM Page 1 DSD A 112.32 Cover 2021.qxp_Layout 1 5/11/21 2:06 PM Page 3 Illinois continues to be a national leader in traffic safety. Over the last decade, traffic fatalities in our state have declined significantly. This is due in large part to innovative efforts to combat drunk and distracted driving, as well as stronger guidelines for new teen drivers. The driving public’s increased awareness and avoidance of hazardous driving behaviors are critical for Illinois to see a further decline in traffic fatalities. Beginning May 3, 2023, the federal government will require your driver’s license or ID card (DL/ID) to be REAL ID compliant for use as identification to board domestic flights. Not every person needs a REAL ID card, which is why we offer you a choice. You decide if you need a REAL ID or standard DL/ID. More information is available on the following pages. The application process for a REAL ID-compliant DL/ID requires enhanced security measures that meet mandated federal guidelines. As a result, you must provide documentation confirming your identity, Social Security number, residency and signature. Please note there is no immediate need to apply for a REAL ID- compliant DL/ID. Current Illinois DL/IDs will be accepted to board domestic flights until May 3, 2023. For more information about the REAL ID program, visit REALID.ilsos.gov or call 833-503-4074. As Secretary of State, I will continue to maintain the highest standards when it comes to traffic safety and public service in Illinois. -
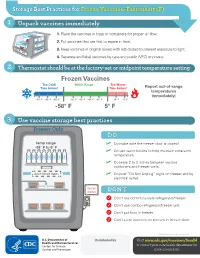
Storage Best Practices for Frozen Vaccines-Fahrenheit
Storage Best Practices for Frozen Vaccines–Fahrenheit (F) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -70° F -65° F -60° F -50° F -45° F -40° F -35° F 10° F 15° F -58° F 5° F 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -58° F to 5° F ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision December 2020 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. Stand-alone D. -

LOST the Official Show Auction
LOST | The Auction 156 1-310-859-7701 Profiles in History | August 21 & 22, 2010 572. JACK’S COSTUME FROM THE EPISODE, “THERE’S NO 574. JACK’S COSTUME FROM PLACE LIKE HOME, PARTS 2 THE EPISODE, “EGGTOWN.” & 3.” Jack’s distressed beige Jack’s black leather jack- linen shirt and brown pants et, gray check-pattern worn in the episode, “There’s long-sleeve shirt and blue No Place Like Home, Parts 2 jeans worn in the episode, & 3.” Seen on the raft when “Eggtown.” $200 – $300 the Oceanic Six are rescued. $200 – $300 573. JACK’S SUIT FROM THE EPISODE, “THERE’S NO PLACE 575. JACK’S SEASON FOUR LIKE HOME, PART 1.” Jack’s COSTUME. Jack’s gray pants, black suit (jacket and pants), striped blue button down shirt white dress shirt and black and gray sport jacket worn in tie from the episode, “There’s Season Four. $200 – $300 No Place Like Home, Part 1.” $200 – $300 157 www.liveauctioneers.com LOST | The Auction 578. KATE’S COSTUME FROM THE EPISODE, “THERE’S NO PLACE LIKE HOME, PART 1.” Kate’s jeans and green but- ton down shirt worn at the press conference in the episode, “There’s No Place Like Home, Part 1.” $200 – $300 576. JACK’S SEASON FOUR DOCTOR’S COSTUME. Jack’s white lab coat embroidered “J. Shephard M.D.,” Yves St. Laurent suit (jacket and pants), white striped shirt, gray tie, black shoes and belt. Includes medical stetho- scope and pair of knee reflex hammers used by Jack Shephard throughout the series. -

CDC Vaccine Storage and Handling Guide
Table of Contents General Information Vaccine Storage and Handling Best Practices 5 Selected Biologicals Diphtheria Toxoid-, Tetanus Toxoid- and acellular Pertussis-Containing Vaccines DTaP: DAPTACEL, Infanrix, Tripedia 11 DTaP-IPV: KINRIX 11 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV/Hib: Pentacel 11 Haemophilus influenzae type b-Containing Vaccines Hib: ActHIB, Hiberix, PedvaxHIB 15 Hib-HepB: Comvax 15 DTaP-IPV/Hib: Pentacel 11 Hepatitis-Containing Vaccines HepA: Havrix, VAQTA 19 HepB: Engerix-B, Recombivax HB 19 HepA-HepB: Twinrix 19 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-HepB-IPV: Pediarix 11 Hib-HepB: Comvax 15 Human Papillomavirus Vaccines HPV2: Cervarix 23 HPV4: Gardasil 23 Vaccine Storage and Handling Guide —————————————————————————————— Page 2 Table of Contents Influenza Vaccines LAIV: FluMist 27 TIV: Afluria, Fluarix, FluLaval, Fluvirin, Fluzone, Fluzone High-Dose, Fluzone Intradermal 29 Measles-, Mumps- and Rubella-Containing Vaccine MMR: M-M-RII 33 MMRV: ProQuad 69 Meningococcal Vaccines MCV4: Menactra, Menveo 37 MPSV4: Menomune 41 Pneumococcal Vaccines PCV13: Prevnar 13 45 PPSV23: Pneumovax 23 45 Poliovirus-Containing Vaccine IPV: IPOL 49 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV: KINRIX 11 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-IPV/Hib: Pentacel 11 Rotavirus Vaccines RV1: ROTARIX 53 RV5: RotaTeq 53 Tetanus Toxoid Vaccine TT: Tetanus Toxoid 57 Vaccine Storage and Handling Guide ——————————————————————————————