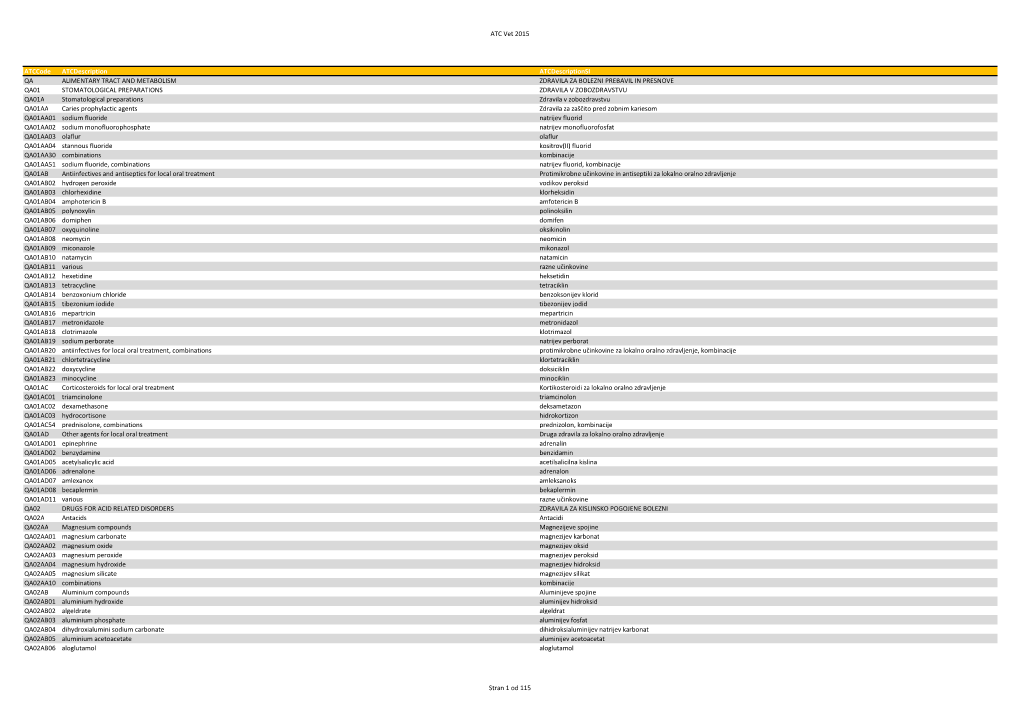
ATC Vet 2015 Atccode Atcdescription Atcdescriptionsi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Full Text in Pdf Format
DISEASES OF AQUATIC ORGANISMS Published July 30 Dis Aquat Org Oral pharmacological treatments for parasitic diseases of rainbow trout Oncorhynchus mykiss. 11: Gyrodactylus sp. J. L. Tojo*, M. T. Santamarina Department of Microbiology and Parasitology, Laboratory of Parasitology, Faculty of Pharmacy, Universidad de Santiago de Compostela, E-15706 Santiago de Compostela, Spain ABSTRACT: A total of 24 drugs were evaluated as regards their efficacy for oral treatment of gyro- dactylosis in rainbow trout Oncorhj~nchusmykiss. In preliminary trials, all drugs were supplied to infected fish at 40 g per kg of feed for 10 d. Twenty-two of the drugs tested (aminosidine, amprolium, benznidazole, b~thionol,chloroquine, diethylcarbamazine, flubendazole, levamisole, mebendazole, n~etronidazole,mclosamide, nitroxynil, oxibendazole, parbendazole, piperazine, praziquantel, roni- dazole, secnidazole, tetramisole, thiophanate, toltrazuril and trichlorfon) were ineffective Triclabenda- zole and nitroscanate completely eliminated the infection. Triclabendazole was effective only at the screening dosage (40 g per kg of feed for 10 d), while nitroscanate was effective at dosages as low as 0.6 g per kg of feed for 1 d. KEY WORDS: Gyrodactylosis . Rainbow trout Treatment. Drugs INTRODUCTION to the hooks of the opisthohaptor or to ulceration as a result of feeding by the parasite. The latter is the most The monogenean genus Gyrodactylus is widespread, serious. though some individual species have a restricted distri- Transmission takes place largely as a result of direct bution. Gyrodactyloses affect numerous freshwater contact between live fishes, though other pathways species including salmonids, cyprinids and ornamen- (contact between a live fish and a dead fish, or with tal fishes, as well as marine fishes including gadids, free-living parasites present in the substrate, or with pleuronectids and gobiids. -

Benznidazole(Rinn)
830 Antiprotozoals Uses and Administration in endemic areas. It may also be used for prophylaxis in non- Neth.: Wellvone; Port.: Wellvone; S.Afr.: Wellvone; Spain: Wellvone; Atovaquone is a hydroxynaphthoquinone antiprotozo- immune travellers9,10 and appears to be well tolerated.10,11 Swed.: Wellvone; Switz.: Wellvone; UK: Wellvone; USA: Mepron. 1. Chiodini PL, et al. Evaluation of atovaquone in the treatment of Multi-ingredient: Austral.: Malarone; Austria: Malarone; Promal; Belg.: al that is also active against the fungus Pneumocystis patients with uncomplicated Plasmodium falciparum malaria. J Malarone; Canad.: Malarone; Cz.: Malarone; Denm.: Malarone; Fr.: Ma- jirovecii. It is used in the treatment and prophylaxis of Antimicrob Chemother 1995; 36; 1073–5. larone; Ger.: Malarone; Gr.: Malarone; Hong Kong: Malarone; Hung.: Ma- larone; Irl.: Malarone; Israel: Malarone; Ital.: Malarone; Malaysia: Malar- pneumocystis pneumonia in patients unable to tolerate 2. Looareesuwan S, et al. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of one; Neth.: Malarone; Norw.: Malarone; NZ: Malarone; Pol.: Malarone; co-trimoxazole, and with proguanil in the treatment acute uncomplicated malaria in Thailand. Am J Trop Med Hyg S.Afr.: Malanil; Singapore: Malarone; Spain: Malarone; Swed.: Malarone; Switz.: Malarone; UK: Malarone; USA: Malarone. and prophylaxis of malaria. 1996; 54: 62–6. 3. Radloff PD, et al. Atovaquone and proguanil for Plasmodium In the treatment of mild to moderate pneumocystis falciparum malaria. Lancet 1996; 347: 1511–14. pneumonia, atovaquone is given orally in a dose of 4. Sabchareon A, et al. Efficacy and pharmacokinetics of atovaquone and proguanil in children with multidrug-resistant Azanidazole (BAN, USAN, rINN) 750 mg with food twice daily as a suspension, for 21 Plasmodium falciparum malaria. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group R01 (Nasal preparations) Table of Contents Page INTRODUCTION 5 DISCLAIMER 7 GLOSSARY OF TERMS USED IN THIS DOCUMENT 8 ACTIVE SUBSTANCES Cyclopentamine (ATC: R01AA02) 10 Ephedrine (ATC: R01AA03) 11 Phenylephrine (ATC: R01AA04) 14 Oxymetazoline (ATC: R01AA05) 16 Tetryzoline (ATC: R01AA06) 19 Xylometazoline (ATC: R01AA07) 20 Naphazoline (ATC: R01AA08) 23 Tramazoline (ATC: R01AA09) 26 Metizoline (ATC: R01AA10) 29 Tuaminoheptane (ATC: R01AA11) 30 Fenoxazoline (ATC: R01AA12) 31 Tymazoline (ATC: R01AA13) 32 Epinephrine (ATC: R01AA14) 33 Indanazoline (ATC: R01AA15) 34 Phenylephrine (ATC: R01AB01) 35 Naphazoline (ATC: R01AB02) 37 Tetryzoline (ATC: R01AB03) 39 Ephedrine (ATC: R01AB05) 40 Xylometazoline (ATC: R01AB06) 41 Oxymetazoline (ATC: R01AB07) 45 Tuaminoheptane (ATC: R01AB08) 46 Cromoglicic Acid (ATC: R01AC01) 49 2 Levocabastine (ATC: R01AC02) 51 Azelastine (ATC: R01AC03) 53 Antazoline (ATC: R01AC04) 56 Spaglumic Acid (ATC: R01AC05) 57 Thonzylamine (ATC: R01AC06) 58 Nedocromil (ATC: R01AC07) 59 Olopatadine (ATC: R01AC08) 60 Cromoglicic Acid, Combinations (ATC: R01AC51) 61 Beclometasone (ATC: R01AD01) 62 Prednisolone (ATC: R01AD02) 66 Dexamethasone (ATC: R01AD03) 67 Flunisolide (ATC: R01AD04) 68 Budesonide (ATC: R01AD05) 69 Betamethasone (ATC: R01AD06) 72 Tixocortol (ATC: R01AD07) 73 Fluticasone (ATC: R01AD08) 74 Mometasone (ATC: R01AD09) 78 Triamcinolone (ATC: R01AD11) 82 -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

(12) Patent Application Publication (10) Pub. No.: US 2001/0053775 A1 Seidel Et Al
US 2001.0053775A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2001/0053775 A1 Seidel et al. 43) Pub. Date: Dec. 20,9 2001 (54) NASAL SOLUTIONS (30) Foreign Application Priority Data (76) Inventors: Matthias Seidel, Ittigen (CH); Jan. 30, 1998 (EP)........................................ 9881OO69-9 Christopher Buckley, Commugny (CH) Publication Classification Correspondence Address: RVNSporation (51) Int. Cl." ......................... A61K 31156; A61K 31/57; ENNINEMARK DEPT (52) U.S. Cl. ........................"I,...s. SUMMIT, NJ 079011027 (21) Appl. No.: 09/880,678 (57) ABSTRACT (22) Filed: Jun. 13, 2001 Related U.S. Application Data The invention relates to liquid pharmaceutical compositions adapted to nasal administration. The liquid nasal formula (63) Continuation of application No. 09/601,123, filed on tions of the invention are characterized inter alia by having Jul. 27, 2000. excellent and prolonged moisturizing properties. US 2001/0053775 A1 Dec. 20, 2001 NASAL SOLUTIONS embodiment of the invention they may also be present in a 0001. The present invention relates to pharmaceutical Slightly Viscous form, e.g. like a Syrup. However, they all compositions intended for nasal administration. More Spe can be clearly discriminated from nasal formulations in gel cifically, it concerns liquid nasal formulations with form in that they-in contrast to gels-are able to form improved moisturizing properties. drops and can be used as SprayS. 0014) Active substances suitable for nasal administration 0002 The nasal administration of active substances is a (a) are e.g. vasoconstrictors, e.g. Xylometazoline, e.g. widely used method of treatment. Active substances which Xylometazoline hydrochloride; indanazoline, metizoline; come into consideration are, for example, vasoconstrictors, naphazoline, e.g. -

Epinephrine Versus Tramazoline to Reduce Nasal Bleeding During Nasotracheal Intubation. a Double- Blind Randomized Trial
Epinephrine versus tramazoline to reduce nasal bleeding during nasotracheal intubation. A double- blind randomized trial Aiji Sato(Boku) ( [email protected] ) Aichi Gakuin University Yoshiki Sento Nagoya City University Yuji Kamimura Nagoya City University Eisuke Kako Nagoya City University Masahiro Okuda Aichi Gakuin University Naoko Tachi Aichi Gakuin University Yoko Okumura Aichi Gakuin University Mayumi Hashimoto Aichi Gakuin University Hiroshi Hoshijima Tohoku University Fumihito Suzuki Akita National Hospital Kazuya Sobue Nagoya City University Research Article Keywords: Nasotracheal intubation, Epinephrine, Tramazoline, Nasal bleeding Posted Date: February 10th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-148779/v1 Page 1/14 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 2/14 Abstract BACKGROUND Nasal bleeding is the most common complication during nasotracheal intubation (NTI). To reduce nasal bleeding, the nasal cavity is treated with vasoconstrictors (epinephrine [E] or tramazoline [T]) prior to NTI. This study aimed to determine whether E or T is more effective and safe for reducing nasal bleeding during NTI. METHODS This study was preregistered on UMIN-CTR after being approved by the IRB of the School of Dentistry at Aichi Gakuin University. Written consent was received from all the patients. Total 206 patients aged 20–70 years and classied as 1–2 on American Society of Anesthesiologists-physical status were scheduled to undergo general anesthesia with NTI. Patients with a narrowed nasal cavity observed during preoperative CT test (n = 3), patients with hypertension (n = 3), patients undergoing antithrombotic therapy, and patients who did not give consent (n = 3) were excluded from the study. -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

UFC PROHIBITED LIST Effective June 1, 2021 the UFC PROHIBITED LIST
UFC PROHIBITED LIST Effective June 1, 2021 THE UFC PROHIBITED LIST UFC PROHIBITED LIST Effective June 1, 2021 PART 1. Except as provided otherwise in PART 2 below, the UFC Prohibited List shall incorporate the most current Prohibited List published by WADA, as well as any WADA Technical Documents establishing decision limits or reporting levels, and, unless otherwise modified by the UFC Prohibited List or the UFC Anti-Doping Policy, Prohibited Substances, Prohibited Methods, Specified or Non-Specified Substances and Specified or Non-Specified Methods shall be as identified as such on the WADA Prohibited List or WADA Technical Documents. PART 2. Notwithstanding the WADA Prohibited List and any otherwise applicable WADA Technical Documents, the following modifications shall be in full force and effect: 1. Decision Concentration Levels. Adverse Analytical Findings reported at a concentration below the following Decision Concentration Levels shall be managed by USADA as Atypical Findings. • Cannabinoids: natural or synthetic delta-9-tetrahydrocannabinol (THC) or Cannabimimetics (e.g., “Spice,” JWH-018, JWH-073, HU-210): any level • Clomiphene: 0.1 ng/mL1 • Dehydrochloromethyltestosterone (DHCMT) long-term metabolite (M3): 0.1 ng/mL • Selective Androgen Receptor Modulators (SARMs): 0.1 ng/mL2 • GW-1516 (GW-501516) metabolites: 0.1 ng/mL • Epitrenbolone (Trenbolone metabolite): 0.2 ng/mL 2. SARMs/GW-1516: Adverse Analytical Findings reported at a concentration at or above the applicable Decision Concentration Level but under 1 ng/mL shall be managed by USADA as Specified Substances. 3. Higenamine: Higenamine shall be a Prohibited Substance under the UFC Anti-Doping Policy only In-Competition (and not Out-of- Competition). -

Wo 2010/075090 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 1 July 2010 (01.07.2010) WO 2010/075090 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C07D 409/14 (2006.01) A61K 31/7028 (2006.01) kind of national protection available): AE, AG, AL, AM, C07D 409/12 (2006.01) A61P 11/06 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US2009/068073 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 15 December 2009 (15.12.2009) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, (26) Publication Language: English TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/122,478 15 December 2008 (15.12.2008) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (71) Applicant (for all designated States except US): AUS- ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, PEX PHARMACEUTICALS, INC. -

Pharmacology on Your Palms CLASSIFICATION of the DRUGS
Pharmacology on your palms CLASSIFICATION OF THE DRUGS DRUGS FROM DRUGS AFFECTING THE ORGANS CHEMOTHERAPEUTIC DIFFERENT DRUGS AFFECTING THE NERVOUS SYSTEM AND TISSUES DRUGS PHARMACOLOGICAL GROUPS Drugs affecting peripheral Antitumor drugs Drugs affecting the cardiovascular Antimicrobial, antiviral, Drugs affecting the nervous system Antiallergic drugs system antiparasitic drugs central nervous system Drugs affecting the sensory Antidotes nerve endings Cardiac glycosides Antibiotics CNS DEPRESSANTS (AFFECTING THE Antihypertensive drugs Sulfonamides Analgesics (opioid, AFFERENT INNERVATION) Antianginal drugs Antituberculous drugs analgesics-antipyretics, Antiarrhythmic drugs Antihelminthic drugs NSAIDs) Local anaesthetics Antihyperlipidemic drugs Antifungal drugs Sedative and hypnotic Coating drugs Spasmolytics Antiviral drugs drugs Adsorbents Drugs affecting the excretory system Antimalarial drugs Tranquilizers Astringents Diuretics Antisyphilitic drugs Neuroleptics Expectorants Drugs affecting the hemopoietic system Antiseptics Anticonvulsants Irritant drugs Drugs affecting blood coagulation Disinfectants Antiparkinsonian drugs Drugs affecting peripheral Drugs affecting erythro- and leukopoiesis General anaesthetics neurotransmitter processes Drugs affecting the digestive system CNS STIMULANTS (AFFECTING THE Anorectic drugs Psychomotor stimulants EFFERENT PART OF THE Bitter stuffs. Drugs for replacement therapy Analeptics NERVOUS SYSTEM) Antiacid drugs Antidepressants Direct-acting-cholinomimetics Antiulcer drugs Nootropics (Cognitive -

Workbook Psychiatry and Narcology
Kharkiv National Medical University Department of Psychiatry, Narcology and Medical Psychology WORKBOOK MANUAL FOR INDIVIDUAL WORK FOR MEDICAL STUDENTS PSYCHIATRY AND NARCOLOGY (Part 2) Student ___________________________________________________________ Faculty _________________________________________________________ Course _________________ Group _____________________________________ Kharkiv 2019 Затверджено вченою радою ХНМУ Протокол №5 від 23.05.2019 р. Psychiatry (Part 2) : workbook manual for individual work of students / I. Strelnikova, G. Samardacova, К. Zelenska – Kharkiv, 2019. – 103 p. Копіювання для розповсюдження в будь-якому вигляді частин або повністю можливо тільки з дозволу авторів навчального посібника. CLASS 7. NEUROTIC DISORDERS. CLINICAL FORMS. TREATMENT AND REHABILITATION. POSTTRAUMATIC STRESS DISORDER. TREATMENT AND REHABILITATION. Psychogenic diseases are a large and clinically varied group of diseases resulting from an effect of acute or long-term psychic traumas, which manifest themselves by both mental and somatoneurological disorders and, as a rule, are reversible. Psychogenic diseases are caused by a psychic trauma, i.e. some events which affect significant aspects of existence of the human being and result in deep psychological feelings. These may be subjectively significant events, i.e. those which are pathogenic for the majority of people. Besides, the psyche may be traumatized by conventionally pathogenic events, which cause feelings in an individual because of his peculiar hierarchy of values. Unfavorable psychogenic effects on the human being cause stress in him, i.e. a nonspecific reaction at the physiological, psychological and behavioural levels. Stress may exert some positive, mobilizing influence, but may result in disorganization of the organism activity. The stress, which exerts a negative influence and causes various disturbances and even diseases, is termed distress. Classification of neurotic disorders I.