Carla Brown Phd Thesis V5
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prayer Cards | Joshua Project
Pray for the Nations Pray for the Nations Abdul in India Aghori in India Population: 35,000 Population: 69,000 World Popl: 66,200 World Popl: 69,000 Total Countries: 3 Total Countries: 1 People Cluster: South Asia Muslim - other People Cluster: South Asia Hindu - other Main Language: Urdu Main Language: Hindi Main Religion: Islam Main Religion: Hinduism Status: Unreached Status: Unreached Evangelicals: 0.00% Evangelicals: 0.00% Chr Adherents: 0.00% Chr Adherents: 0.00% Scripture: Complete Bible Scripture: Complete Bible www.joshuaproject.net www.joshuaproject.net Source: Isudas Source: AKS.9955 "Declare his glory among the nations." Psalm 96:3 "Declare his glory among the nations." Psalm 96:3 Pray for the Nations Pray for the Nations Ansari in India Asur in India Population: 10,700,000 Population: 32,000 World Popl: 14,792,500 World Popl: 33,200 Total Countries: 6 Total Countries: 2 People Cluster: South Asia Muslim - Ansari People Cluster: South Asia Tribal - other Main Language: Urdu Main Language: Asuri Main Religion: Islam Main Religion: Hinduism Status: Unreached Status: Minimally Reached Evangelicals: Unknown % Evangelicals: Unknown % Chr Adherents: 0.00% Chr Adherents: 8.47% Scripture: Complete Bible Scripture: Unspecified www.joshuaproject.net www.joshuaproject.net Source: Biswarup Ganguly "Declare his glory among the nations." Psalm 96:3 "Declare his glory among the nations." Psalm 96:3 Pray for the Nations Pray for the Nations Badhai (Hindu traditions) in India Baidya (Hindu traditions) in India Population: 6,549,000 Population: -

CENTRAL LIST of OTHER BACKWARD CLASSES Sl
CENTRAL LIST OF OTHER BACKWARD CLASSES Sl.No. Name of the Castes/Sub-castes/Synonyms/ Entry No. in the Communities Central List BIHAR 1 Abdal 1 2 Agariya 2 3 Aghori 3 4 Amaat 4 5 Bagdi 77 6 Bakho (Muslim) 130 7 Banpar 113 8 Barai 82 9 Barhai (Viswakarma) 81 10 Bari 78 11 Beldar 79 12 Bhar 85 13 Bhaskar 86 14 Bhat, Bhatt 88 15 Bhathiara (Muslim) 84 16 Bind 80 17 Bhuihar, Bhuiyar 87 18 Chain, Chayeen 39 19 Chapota 40 20 Chandrabanshi (Kahar) 41 21 Chanou 43 22 Chik (Muslim) 38 23 Christian converts from Other Backward Classes 121 24 Christian converts from Scheduled Castes 120 25 Churihar (Muslim) 42 26 Dafali (Muslim) 46 27 Dangi 123 28 Devhar 55 29 Dhamin 59 30 Dhanuk 56 31 Dhanwar 122 32 Dhankar 60 33 Dhekaru 47 34 Dhimar 61 35 Dhobi (Muslim) 57 36 Dhunia (Muslim) 58 37 Gaddi 30 38 Gandarbh or Gandharb 31 39 Gangai (Ganesh) 32 40 Gangota, Gangoth 33 41 Ghatwar 37 42 Godi (Chhava) 29 43 Gorh, Gonrh (only in the district of Saran & Rohtas) 34 44 Goud 36 45 Gulgaliya 35 46 Idrisi or Darzi (Muslim) 119 47 Jogi (Jugi) 44 48 Kadar 7 49 Kaivartta/Kaibartta 8 50 Kagzi 16 51 Kalandar 9 52 Kalwar 124(a) Kalal, Eraqui 124(b) 53 Kamar (Lohar, Karmakar, Visvakarma) 18 54 Kanu 17 55 Kapadia 20 56 Kasab (Kasai) (Muslim) 5 57 Kaura 10 58 Kawar 11 59 Kewat 6 Keot 60 Khadwar (only in the district of Sivan and Rohtas) 26 61 Khangar 23 62 Khatik 22 63 Khatwa 24 64 Khatwe 25 65 Khelta 28 66 Khetauri, Khatauri 27 67 Kochh 12 68 Korku 13 69 Kosta, Koshta 21 70 Kumarbhag Pahadia 14 71 Kulahia 125 72 Kurmi 15 Kurmi (Mahto) (in Chhotanagpur Division only) 73 -

*‡Table 5. Ethnic and National Groups
T5 Table[5.[Ethnic[and[National[Groups T5 T5 TableT5[5. [DeweyEthnici[Decimaand[NationalliClassification[Groups T5 *‡Table 5. Ethnic and National Groups The following numbers are never used alone, but may be used as required (either directly when so noted or through the interposition of notation 089 from Table 1) with any number from the schedules, e.g., civil and political rights (323.11) of Navajo Indians (—9726 in this table): 323.119726; ceramic arts (738) of Jews (—924 in this table): 738.089924. They may also be used when so noted with numbers from other tables, e.g., notation 174 from Table 2 In this table racial groups are mentioned in connection with a few broad ethnic groupings, e.g., a note to class Blacks of African origin at —96 Africans and people of African descent. Concepts of race vary. A work that emphasizes race should be classed with the ethnic group that most closely matches the concept of race described in the work Except where instructed otherwise, and unless it is redundant, add 0 to the number from this table and to the result add notation 1 or 3–9 from Table 2 for area in which a group is or was located, e.g., Germans in Brazil —31081, but Germans in Germany —31; Jews in Germany or Jews from Germany —924043. If notation from Table 2 is not added, use 00 for standard subdivisions; see below for complete instructions on using standard subdivisions Notation from Table 2 may be added if the number in Table 5 is limited to speakers of only one language even if the group discussed does not approximate the whole of the -

0 Acknowledgements.Pmd
Epidemiological and entomological aspects of an outbreak of chikungunya in Lakshadweep Islands, India, during 2007 R.S. Sharmaa#, M.K. Showkath Alib, G.P.S. Dhillona aNational Vector-Borne Disease Control Programme, Delhi – 110 054, India bNational Institute of Communicable Diseases, Kozhikode, Kerala, India Abstract Since 2006, the Indian state of Kerala has reported outbreaks of chikungunya (CHIK). During July- August 2007, an unusual increase in the incidence of fever was noticed in Kadmat, Amini and Kavaratti Islands in the Union Territory of Lakshwadeep, a group of Indian islands adjacent to the Kerala coast in the Arabian Sea. The populations affected as per the primary health centre (PHC) records of three islands, viz. Kadmat, Amini and Kavaratti, was 85%, 1.4% and 0.15% respectively. Entomological surveys revealed very high larval indices of Aedes albopictus only in the three surveyed islands. Aedes aegypti, the classical vector of dengue, was not detected. The maximum breeding of Ae. albopictus was found in coconut shells (57%), tyres (9%), metal containers (9%) and plastic containers (8%). The breeding was also detected in tree holes and rat-bitten coconuts on top of the trees. The House Index for Ae. albopictus ranged between 95.4% in Kavaratti to 79% in Amini. Kadmat island which was the worst affected, recording the maximum Container Index of 90%, compared with 40% in Amini island. The CHIK outbreak seemed to have been caused by importation of the virus from Kerala, because of heavy movement of the islanders to the mainland. Keywords: Chikungunya; Aedes albopictus; Lakshadweep Islands. Introduction Rajahmundry, Vishakpatnam and Kakinada in 1965. -

An Analytical Study of Women-Related Verses of S¯Ura An-Nisa
Gunawan Adnan Women and The Glorious QurÞÁn: An Analytical Study of Women-RelatedVerses of SÙra An-NisaÞ erschienen in der Reihe der Universitätsdrucke des Universitätsverlages Göttingen 2004 Gunawan Adnan Women and The Glorious QurÞÁn: An Analytical Study of Women- RelatedVerses of SÙra An-NisaÞ Universitätsdrucke Göttingen 2004 Die Deutsche Bibliothek – CIP-Einheitsaufnahme Ein Titelsatz für diese Publikation ist bei der Deutschen Bibliothek erhältlich. © Alle Rechte vorbehalten, Universitätsverlag Göttingen 2004 ISBN 3-930457-50-4 Respectfully dedicated to My honorable parents ...who gave me a wonderful world. To my beloved wife, son and daughter ...who make my world beautiful and meaningful as well. i Acknowledgements All praises be to AllÁh for His blessing and granting me the health, strength, ability and time to finish the Doctoral Program leading to this book on the right time. I am indebted to several persons and institutions that made it possible for this study to be undertaken. My greatest intellectual debt goes to my academic supervisor, Doktorvater, Prof. Tilman Nagel for his invaluable advice, guidance, patience and constructive criticism throughout the various stages in the preparation of this dissertation. My special thanks go to Prof. Brigitta Benzing and Prof. Heide Inhetveen whose interests, comments and guidance were of invaluable assistance. The Seminar for Arabic of Georg-August University of Göttingen with its international reputation has enabled me to enjoy a very favorable environment to expand my insights and experiences especially in the themes of Islamic studies, literature, phylosophy, philology and other oriental studies. My thanks are due to Dr. Abdul RazzÁq Weiss who provided substantial advice and constructive criticism for the perfection of this dissertation. -

A Curriculum to Prepare Pastors for Tribal Ministry in India
Andrews University Digital Commons @ Andrews University Dissertation Projects DMin Graduate Research 2007 A Curriculum To Prepare Pastors for Tribal Ministry in India Calvin N. Joshua Andrews University Follow this and additional works at: https://digitalcommons.andrews.edu/dmin Part of the Practical Theology Commons Recommended Citation Joshua, Calvin N., "A Curriculum To Prepare Pastors for Tribal Ministry in India" (2007). Dissertation Projects DMin. 612. https://digitalcommons.andrews.edu/dmin/612 This Project Report is brought to you for free and open access by the Graduate Research at Digital Commons @ Andrews University. It has been accepted for inclusion in Dissertation Projects DMin by an authorized administrator of Digital Commons @ Andrews University. For more information, please contact [email protected]. ABSTRACT A CURRICULUM TO PREPARE PASTORS FOR TRIBAL MINISTRY IN INDIA by Calvin N. Joshua Adviser: Bruce L. Bauer ABSTRACT OF GRADUATE STUDENT RESEARCH Dissertation Andrews University Seventh-day Adventist Theological Seminary Title: A CURRICULUM TO PREPARE PASTORS FOR TRIBAL MINISTRY IN INDIA Name of researcher: Calvin N. Joshua Name and degree of faculty adviser: Bruce L. Bauer, DMiss. Date Completed: September 2007 Problem The dissertation project establishes the existence of nearly one hundred million tribal people who are forgotten but continue to live in human isolation from the main stream of Indian society. They have their own culture and history. How can the Adventist Church make a difference in reaching them? There is a need for trained pastors in tribal ministry who are culture sensitive and knowledgeable in missiological perspectives. Method Through historical, cultural, religious, and political analysis, tribal peoples and their challenges are identified. -

Caste, Kinship and Sex Ratios in India
NBER WORKING PAPER SERIES CASTE, KINSHIP AND SEX RATIOS IN INDIA Tanika Chakraborty Sukkoo Kim Working Paper 13828 http://www.nber.org/papers/w13828 NATIONAL BUREAU OF ECONOMIC RESEARCH 1050 Massachusetts Avenue Cambridge, MA 02138 March 2008 We thank Bob Pollak, Karen Norberg, David Rudner and seminar participants at the Work, Family and Public Policy workshop at Washington University for helpful comments and discussions. We also thank Lauren Matsunaga and Michael Scarpati for research assistance and Cassie Adcock and the staff of the South Asia Library at the University of Chicago for their generous assistance in data collection. We are also grateful to the Weidenbaum Center and Washington University (Faculty Research Grant) for research support. The views expressed herein are those of the author(s) and do not necessarily reflect the views of the National Bureau of Economic Research. NBER working papers are circulated for discussion and comment purposes. They have not been peer- reviewed or been subject to the review by the NBER Board of Directors that accompanies official NBER publications. © 2008 by Tanika Chakraborty and Sukkoo Kim. All rights reserved. Short sections of text, not to exceed two paragraphs, may be quoted without explicit permission provided that full credit, including © notice, is given to the source. Caste, Kinship and Sex Ratios in India Tanika Chakraborty and Sukkoo Kim NBER Working Paper No. 13828 March 2008 JEL No. J12,N35,O17 ABSTRACT This paper explores the relationship between kinship institutions and sex ratios in India at the turn of the twentieth century. Since kinship rules varied by caste, language, religion and region, we construct sex-ratios by these categories at the district-level using data from the 1901 Census of India for Punjab (North), Bengal (East) and Madras (South). -

Downloaded on 2017-02-12T13:48:37Z
Title Synthesis and evaluation of novel quinolines and quinazolinediones as potential anti-cancer agents Author(s) Greaney, Kieran Publication date 2014 Original citation Greaney, K. 2014. Synthesis and evaluation of novel quinolines and quinazolinediones as potential anti-cancer agents. PhD Thesis, University College Cork. Type of publication Doctoral thesis Rights © 2014, Kieran Greaney. http://creativecommons.org/licenses/by-nc-nd/3.0/ Embargo information Please note that Chapters 4-8 (pp.87-262) and Appendices (pp.I-XXIII) are unavailable due to a restriction requested by the author. Embargo lift date 2018-11-05T12:28:56Z Item downloaded http://hdl.handle.net/10468/2042 from Downloaded on 2017-02-12T13:48:37Z Synthesis and Evaluation of Novel Quinolines and Quinazolinediones as Potential Anti-Cancer Agents Kieran Greaney, B.Sc. Thesis presented for the degree of Doctor of Philosophy to National University of Ireland, Cork. Department of Chemistry Supervisor: Dr. Florence McCarthy Head of Department: Prof. Martyn Pemble May 2014 Table of Chapters Acknowledgements iii Declaration iv Abstract vi Abbreviations viii 1.0 Biological Introduction 12 2.0 Quinoline Chemical Introduction 37 3.0 Quinazoline Chemical Introduction 62 4.0 Aims and Objectives 87 5.0 Chemical Results and Discussion 94 6.0 Biological Results and Discussion 178 7.0 Current Perspectives 196 8.0 Experimental 204 9.0 References 263 Appendices Acknowledgements To express my thanks through words seems such an injustice but I’ll give it my best shot. First and foremost I would like to express my deepest gratitude to Dr. Florence McCarthy whose guidance and support during this project has been nothing short of immense, and for having me as part of such a great research group. -
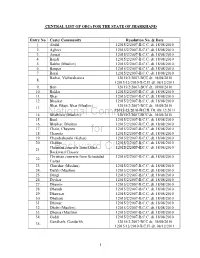
1 CENTRAL LIST of Obcs for the STATE of JHARKHAND Entry No
CENTRAL LIST OF OBCs FOR THE STATE OF JHARKHAND Entry No Caste/ Community Resolution No. & Date 1. Abdal 12015/2/2007-B.C.C. dt. 18/08/2010 2. Aghori 12015/2/2007-B.C.C. dt. 18/08/2010 3. Amaat 12015/2/2007-B.C.C. dt. 18/08/2010 4. Bagdi 12015/2/2007-B.C.C. dt. 18/08/2010 5. Bakho (Muslim) 12015/2/2007-B.C.C. dt. 18/08/2010 6. Banpar 12015/2/2007-B.C.C. dt. 18/08/2010 7. Barai 12015/2/2007-B.C.C. dt. 18/08/2010 Barhai, Vishwakarma 12015/2/2007-BCC dt. 18/08/2010 8. 12015/13/2010-B.C.II. dt. 08/12/2011 9. Bari 12015/2/2007-BCC dt. 18/08/2010 10. Beldar 12015/2/2007-B.C.C. dt. 18/08/2010 11. Bhar 12015/2/2007-B.C.C. dt. 18/08/2010 12. Bhaskar 12015/2/2007-B.C.C. dt. 18/08/2010 Bhat, Bhatt, Bhat (Muslim) 12015/2/2007-BCC dt. 18/08/2010 13. 12015/13/2010-B.C.II. Dt. 08/12/2011 14. Bhathiara (Muslim) 12015/2/2007-BCC dt. 18/08/2010 15. Bind 12015/2/2007-B.C.C. dt. 18/08/2010 16. Bhuihar, Bhuiyar 12015/2/2007-B.C.C. dt. 18/08/2010 17. Chain, Chayeen 12015/2/2007-B.C.C. dt. 18/08/2010 18. Chapota 12015/2/2007-B.C.C. dt. 18/08/2010 19. Chandrabanshi (Kahar) 12015/2/2007-B.C.C. -

Clans, Tribes and Their Locality in Chechnya, Albania, Afghanistan and Iraq
Appendix Clans, Tribes and Their Locality in Chechnya, Albania, Afghanistan and Iraq While compiling the lists with clans the author found that in some cases lists do not (completely) overlap. Since the sources are trustworthy, they are indicated here. This shows the importance of correct knowledge of clans and their influence in the areas they are inhabiting. 1 Clans in Chechnya1 Confederation Clans Localisation A’kkhiï Bartchakhoï, J’evoï, Ziogoï, In the east of Chechnya, Pkhiartchoï, Pkhiartchakhoï, near Daghestan; North of Nokkhoï, Va’ppiï Daghestan Malkhiï Amkhoï, Bia’stiï, Bienastkhoï, In the south west of Italtchkhoï, Kamalkhoï, Chechnya, along the frontier Kkhoratkhoï, Kiegankhoï, with Ingushetia and Georgia Mechiï, Sakankhoï, Teratkhoï, Tchiarkhoï, Erkhoï, Yamkhoï Nokhtchmakhkoï Aïtkhaloï, Belguiatoï, Benoï, East, Southeast and part of Biltoï, Guandarguenoï, central Chechnya Guiordaloï, Gouonoï, Zandak’oï, Ikhiiroï, Ichkhoï, Kourchaloï, Sessankhoï, Tchermoï, Tsientaroï, Tchartoï, Eguiachbatoï, Enakkhaloï, Enganoï, Chouonoï, Yalkhoï, Yaliroï Terloï Nik’aroï, O’chniï, Cho’ndiï, Along the Tchanty-Argun Eltpkh’arkhoï 1 M.A. Mamakaev. Le taipe (lignee) tchétchène dans la période de sa désintégration (Grozny: Maison d’édition tchétchéno-ingouche, 1973), 18–19 in Viacheslav Avioutskii, 54. © koninklijke brill nv, leiden, ���� | doi:10.1163/9789004415485_013 Charlotte Hille - 9789004415485 Downloaded from Brill.com09/30/2021 01:32:57AM via free access <UN> �36 APPENDIX: CLANS, TRIBES AND THEIR LOCALITY Confederation Clans Localisation -

REVISION of 'Tlfesjjist.'Vof SCHEDULED Ofgtes Anfi
REVISIONv OF 'TlfEsJjIST.'VOf Svv'vr-x'- " -?>-•'. ? ••• '■gc^ ’se v ^ - - ^ r v ■*■ SCHEDULED OfgTES ANfi SCHEDULED-TIBBS' g o VESNMEbrr pF ,i^d£4 .DEI^Ap’MksfT OF.SOCIAL SEmFglTY THE REPORT OF THE ADVISORY COMMITTEE ON THE REVISION OF THE LISTS OF SCHEDULED CASTES AND SCHEDULED TRIBES GOVERNMENT OF INDIA DEPARTMENT OF SOCIAL SECURITY CONTENTS PART I PTER I. I n t r o d u c t i o n ............................................................. 1 II. Principles and P o l i c y .................................................... 4 III. Revision o f L i s t s .............................................................. 12 IV. General R eco m m en d a tio n s.......................................... 23 V. Appreciation . 25 PART II NDJX I. List of Orders in force under articles 341 and 342 of the Constitution ....... 28 II. Resolution tonstituting the Committee . 29 III, List of persons 'who appeared before the Committee . 31 (V. List of Communities recommended for inclusion 39 V. List of Communities recommended for exclusion 42 VI, List of proposals rejected by the Committee 55 SB. Revised Statewise lists of Scheduled Castes and . Scheduled T r i b e s .................................................... ■115 CONTENTS OF APPENDIX 7 1 i Revised Slantwise Lists pf Scheduled Castes and Scheduled Tribes Sch. Sch. Slate Castes Tribes Page Page Andhra Pracoih .... 52 9i rtssam -. •S'S 92 Bihar .... 64 95 G u j a r a i ....................................................... 65 96 Jammu & Kashmir . 66 98 Kerala............................................................................... 67 98 Madhya Pradesh . 69 99 M a d r a s .................................................................. 71 102 Maharashtra ........................................................ 73 103 Mysore ....................................................... 75 107 Nagaland ....................................................... 108 Oriisa ....................................................... 78 109 Punjab ...... 8i 110 Rejssth&n ...... -

Synthesis of New 2- and 3-Hydroxyquinoline- 4-Carboxylic Acid Derivatives As Potential Antioxidants
DOI 10.1515/hc-2013-0163 Heterocycl. Commun. 2014; 20(2): 81–88 Mohammed A. Massoud, Serry A. El Bialy, Waleed A. Bayoumi* and Walaa M. El Husseiny Synthesis of new 2- and 3-hydroxyquinoline- 4-carboxylic acid derivatives as potential antioxidants Abstract: A new series of 3-aryl-2-hydroxyquinoline- derivatives are 7-chloro-4-hydroxyquinoline (6) and 7-fluoro- 4-carboxylic acids 17a,b, 2-aryl-3-hydroxyquinoline- 4-hydroxyquinoline (7), which have been characterized by 4-carboxylic acids 12a–d and their derivatives 13–16 and their antioxidant effect against free radical initiated peroxi- 18–21 were designed, synthesized and evaluated for their dation [10]. Additional synthetic quinolone derivatives with antioxidant activity using the ABTS assay method. Com- effective antioxidant activity are TA 270 (8) [11] and quin- pounds 14 and 21a,b showed good antioxidant activity, oline-3-carbohydrazides (9) [12] (Figure 1). 2-Substituted whereas the remaining compounds displayed mild to benzimidazoles were recently reported to possess antioxi- moderate activity. All compounds were characterized by dant activity [13]. Based on the previous data and literature physical and spectral data. review, new starting synthons were designed and synthe- sized: 3-aryl-2-hydroxyquinoline-4-carboxylic acids (17a,b) Keywords: ABTS assay; antioxidant; benzimidazole; quin- and 2-aryl-3-hydroxyquinoline-4-carboxylic acids (12a-d). oline-4-carboxylic acid; synthesis. Hybridization with several pharmacophoric moieties were achieved in order to explore the effect on enhancing the antioxidant activity. The following analogs were obtained: *Corresponding author: Waleed A. Bayoumi, Faculty of Pharmacy, ester derivatives ( , and ), O-acetyl derivatives Department of Pharmaceutical Organic Chemistry, 13a-d 15 18a,b Mansoura University, Mansoura 35516, Egypt, (16a-d) and O-alkyl/aralkyl derivatives (19a-d and 20a,b).