Challenges of HPLC Method Development and Validation for the Assay of Bemotrizinol from Complex Matrix in Cosmeceutical Preparation Kallol Jana*, Beduin Mahanti
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GAO-18-61, SUNSCREEN: FDA Reviewed Applications For
United States Government Accountability Office Report to Congressional Committees November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed GAO-18-61 November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed Highlights of GAO-18-61, a report to congressional committees Why GAO Did This Study What GAO Found Using sunscreen as directed with other The Food and Drug Administration (FDA), within the Department of Health and sun protective measures may help Human Services, implemented requirements for reviewing applications for reduce the risk of skin cancer—the sunscreen active ingredients within time frames set by the Sunscreen Innovation most common form of cancer in the Act, which was enacted in November 2014. For example, the agency issued a United States. In the United States, guidance document on safety and effectiveness testing in November 2016. sunscreen is considered an over-the- counter drug, which is a drug available As of August 2017, all applications for sunscreen active ingredients remain to consumers without a prescription. pending after the agency determined more safety and effectiveness data are Some sunscreen active ingredients not needed. By February 2015, FDA completed its initial review of the safety and currently marketed in the United States effectiveness data for each of the eight pending applications, as required by the have been available in products in act. FDA concluded that additional data are needed to determine that the other countries for more than a ingredients are generally recognized as safe and effective (GRASE), which is decade. Companies that manufacture needed so that products using the ingredients can subsequently be marketed in some of these ingredients have sought the United States without FDA’s premarket approval. -

Research Article Development and Validation of a Stability Indicating
Hindawi Publishing Corporation ISRN Chromatography Volume 2013, Article ID 506923, 12 pages http://dx.doi.org/10.1155/2013/506923 Research Article Development and Validation of a Stability Indicating RP-HPLC Method for the Determination of Two Sun Protection Factors (Koptrizon and Tinosorb S) in Topical Pharmaceutical Formulations Using Experimental Designs Chinmoy Roy1,2 and Jitamanyu Chakrabarty2 1 Analytical Research and Development, Dr. Reddy’s Laboratories Ltd., Bachupally, Hyderabad, Andhra Pradesh 500090, India 2 Department of Chemistry, National Institute of Technology, Durgapur, West Bengal 713209, India Correspondence should be addressed to Chinmoy Roy; [email protected] Received 11 March 2013; Accepted 15 April 2013 Academic Editors: C. Akbay and J. A. P. Coelho Copyright © 2013 C. Roy and J. Chakrabarty. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. A novel, simple, validated stability indicating HPLC method was developed for determination of Koptrizon and Tinosorb S. Stability indicating power of the method was established by forced degradation study. The chromatographic separation was achieved with Waters X Bridge C18 column, by using mobile phase consisting of a mixture of acetonitrile : tetrahydrofuran : water (38 : 38 : 24, v/v/v). The method fulfilled validation criteria and was shown to be sensitive, with limits of detection (LOD) and quantitation −1 −1 (LOQ) of 0.024 and 0.08 gmL for Koptrizon and 0.048 and 0.16 gmL for Tinosorb S, respectively. The developed method is validated for parameters like precision, accuracy, linearity, solution stability, specificity, and ruggedness as per ICH norms. -
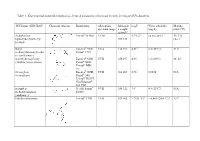
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

Photophysics and Skin Penetration of Active Agents in a Commercial Sunscreen and Insect Repellent
PHOTOPHYSICS AND SKIN PENETRATION OF ACTIVE AGENTS IN A COMMERCIAL SUNSCREEN AND INSECT REPELLENT by DONALD PRETTYPAUL A Dissertation submitted to the Graduate School-Newark Rutgers, The State University of New Jersey In partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Chemistry written under the direction of Professor Richard Mendelsohn Professor Piotr Piotrowiak and approved by Newark, New Jersey October 2018 ©2018 Donald Prettypaul ALL RIGHTS RESERVED ABSTRACT OF DISSERTATION PHOTOPHYSICS AND SKIN PENETRATION OF ACTIVE AGENTS IN A COMMERCIAL SUNSCREEN AND INSECT REPELLENT By DONALD PRETTYPAUL Dissertation co-Directors: Professor Richard Mendelsohn Professor Piotr Piotrowiak This dissertation is focused on active agents in commercial sunscreen and insect repellent products. It consists of two parts, the first focusing on the photophysics of a sunscreen active agent and the second on the permeation and spatial distribution of the sunscreen active and an insect repellent active when these agents are applied to ex-vivo human skin. In the photochemistry study, ultrafast spectroscopy was used to study the excited state dynamics of the sunscreen molecule, Bemotrizinol. The work focused on the dissipation rates of the electronic excitation energy in different solvents. To complement the results from time-resolved femtosecond spectroscopy, Hartree- Fock UH/UHF 6-31G* calculations were used to characterize the ground and excited states potential energy surfaces. The results indicate that the excited state deactivation pathway follows a proton coupled electron transfer process which ii proceeds via a concerted mechanism. The dependencies on solvent polarity, viscosity, and H/D isotope effects, were investigated. Sunscreen products have been developed to protect skin from ultraviolet (UV) radiation; to achieve adequate protection, the sunscreen must be evenly applied and remain on the surface of the skin. -

New Technology Provides Cosmetically Elegant Photoprotection Zoe Diana Draelos, MD; Christian Oresajo; Margarita Yatskayer; Angelike Galdi; Isabelle Hansenne
COSMETIC CONSULTATION New Technology Provides Cosmetically Elegant Photoprotection Zoe Diana Draelos, MD; Christian Oresajo; Margarita Yatskayer; Angelike Galdi; Isabelle Hansenne he primary preventable cause of photoaging is United States, as there is no official rating system for exposure to UVA radiation. This wavelength UVA photoprotection yet. T emitted by the sun is present year round in all Ecamsule absorbs UVA radiation in the range of 320 to latitudes. Currently, the majority of sun-protective prod- 360 nm, but its peak absorbance occurs at 345 nm. It is ucts provide excellent UVB protection with minimal UVA typically combined with other organic sunscreen ingredi- protection. Although UVB protection is important in ents, such as avobenzone and octocrylene. Avobenzone, order to prevent sun damage to the skin from occurring, which is a photounstable photoprotectant, becomes UVA protection is equally important. New developments photostable when combined with octocrylene. These in raw material science have led to the manufacture of ingredients yield a photostable broad-spectrum sunscreen novel ingredients able to provide unprecedented photo- combination. However, the active sunscreen agents are protection COSin the UVA spectrum. DERMonly part of the formulation. Also important in sunscreen One of the most significant developments in cutaneous is the construction of the vehicle to deliver the photo- UVA protection was the discovery of ecamsule (Figure 1), protectants in an aesthetically pleasing manner, enticing also known as Mexoryl SX. Mexoryl SX, which has patients to wear the product. Sunscreens fail to be effec- the International Nomenclature of Cosmetic Ingredients tive if they remain in the bottle. name of terephthalylidene dicamphor sulfonic acid, is In order to evaluate the efficacy and tolerability of a water soluble. -

Effect of 10 UV Filters on the Brine Shrimp Artemia Salina and the Marine Microalgae Tetraselmis Sp
bioRxiv preprint doi: https://doi.org/10.1101/2020.01.30.926451; this version posted January 31, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Effect of 10 UV filters on the brine shrimp Artemia salina and the marine microalgae Tetraselmis sp. Evane Thorel, Fanny Clergeaud, Lucie Jaugeon, Alice M. S. Rodrigues, Julie Lucas, Didier Stien, Philippe Lebaron* Sorbonne Université, CNRS USR3579, Laboratoire de Biodiversité et Biotechnologie Microbienne, LBBM, Observatoire Océanologique, 66650, Banyuls-sur-mer, France. *corresponding author : E-mail address : [email protected] Abstract The presence of pharmaceutical and personal care products’ (PPCPs) residues in the aquatic environment is an emerging issue due to their uncontrolled release, through grey water, and accumulation in the environment that may affect living organisms, ecosystems and public health. The aim of this study is to assess the toxicity of benzophenone-3 (BP-3), bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT), butyl methoxydibenzoylmethane (BM), methylene bis- benzotriazolyl tetramethylbutylphenol (MBBT), 2-Ethylhexyl salicylate (ES), diethylaminohydroxybenzoyl hexyl benzoate (DHHB), diethylhexyl butamido triazone (DBT), ethylhexyl triazone (ET), homosalate (HS), and octocrylene (OC) to marine organisms from two major trophic levels including autotrophs (Tetraselmis sp.) and heterotrophs (Artemia salina). In general, EC50 results show that both HS and OC are the most toxic for our tested species, followed by a significant effect of BM on Artemia salina but only at high concentrations (1 mg/L) and then an effect of ES, BP3 and DHHB on the metabolic activity of the microalgae at 100 µg/L. -

1. Background and Action Requested
June 27, 2019 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Comments Submitted to Docket No. 1978N-0018 (Formerly Docket No. FDA–1978–N– 0038) for “Sunscreen Drug Products for Over-the-Counter Human Use,” Regulatory Information No. 0910-AF43 DSM Nutritional Products LLC (“DSM”) is pleased to provide the following comments in response to the Food and Drug Administration’s (FDA’s) Tentative Final Monograph (“TFM”) for Sunscreen Drug Products Over-the-Counter (OTC) Human Use, published at 84 Fed. Reg. 6204 (February 26, 2019). As a global science-based company active in Nutrition, Health and Sustainable Living, DSM is a world leading supplier of vitamins, feed, food, pharmaceutical, cosmetic, personal care and aroma ingredients. In sunscreens, DSM is a leading world-wide provider of numerous UV filters, including Avobenzone. DSM takes human health and safety very seriously and believes that sunscreens are proven and effective interventions that help provide consumer protection against skin cancers and skin damage caused by the sun’s rays. DSM is committed to proactively supporting the safety and availability of existing and new sunscreen active ingredients that help protect public health. DSM requests that FDA modify its proposed rule taking into consideration the comments provided herein. 1. Background and Action Requested Skin cancer is a significant, and largely preventable, public health concern. Sunscreens have been widely and safely used by the general public for their photoprotective properties, including prevention of photocarcinogenesis and photoaging and management of photodermatoses for more than 40 years. FDA’s Sunscreen Monograph rule has identified the permitted active ingredients (UV-filters) for sunscreens since 1978. -

FDA Proposes Sunscreen Regulation Changes February 2019
FDA Proposes Sunscreen Regulation Changes February 2019 The U.S. Food and Drug Administration (FDA) regulates sunscreens to ensure they meet safety and eectiveness standards. To improve the quality, safety, and eectiveness of sunscreens, FDA issued a proposed rule that describes updated proposed requirements for sunscreens. Given the recognized public health benets of sunscreen use, Americans should continue to use broad spectrum sunscreen with SPF 15 or higher with other sun protective measures as this important rulemaking eort moves forward. Highlights of FDA’s Proposals Sunscreen active ingredient safety and eectiveness Two ingredients (zinc oxide and titanium dioxide) are proposed to be safe and eective for sunscreen use and two (aminobenzoic acid (PABA) and trolamine salicylate) are 1 proposed as not safe and eective for sunscreen use. FDA proposes that it needs more safety information for the remaining 12 sunscreen ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, avobenzone). New proposed sun protection factor Sunscreen dosage forms (SPF) and broad spectrum Sunscreen sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks are requirements 2 proposed as safe and eective. FDA 3 • Raise the maximum proposed labeled SPF proposes that it needs more data for from SPF 50+ to SPF 60+ sunscreen powders. • Require any sunscreen SPF 15 or higher to be broad spectrum • Require for all broad spectrum products SPF 15 and above, as SPF increases, broad spectrum protection increases New proposed label requirements • Include alphabetical listing of active ingredients on the front panel • Require sunscreens with SPF below 15 to include “See Skin Cancer/Skin Aging alert” on the front panel 4 • Require font and placement changes to ensure SPF, broad spectrum, and water resistance statements stand out Sunscreen-insect repellent combination 5 products proposed not safe and eective www.fda.gov. -

Chemical UVR Absorbers
Chemical UVR Absorbers The names given in bold and used Diisopropyl methyl cinnamate Glyceryl ethyihexanoate dimethoxy- throughout this handbook are those of Empirical formula: cinnamate the International Nomenclature of C 6H22O2 Chemical names. Cosmetic Ingredients. Glyceryl octanoate dimethoxycinnamate; Chemical names: 2-propenoic acid, 3-(4-methoxyphenyl)-, 2-Propenoic acid, 3-12,4bis(1 diester with 1 ,3-dihydroxy-2-(2-ethyl-1 - methylethyphenyl-methyl ester; 2,5- oxohexyl)oxypropane diisopropyl methyl cinnamate _ lsoamyl-para-methoxycinnamate Ethyihexyl methoxycinnamate Empirical formula: Empirical formula: C151-12003 C 8H26O3 Chemical names: Cinnamates Chemical names: Amyl4-methoxycinnamate; isopentyl-4- 2-Ethylhexyl-4-methoxycin nam ate; methoxycinnamate; isopenlyl-para- Cinoxate 2-ethyl-hexyl-para-methoxycinnamate; methoxy-cinnamate; 3-(4-methoxyphenyl)- Empirical formula: para-methoxycinnamic acid, 2-ethylhexyl 2-propenoic acid, isopentyl ester Ci4HieO4 ester; 3-(4-methoxyphenyl)-2-propenoic acid, 2-ethylhexyl ester; octinoxate; octyl Trade names: Chemical names: methoxycinnamate; 2-propenoic acid, 3- Neo Heliopan type E 1000; Solarum AMC 2- Ethoxyothyl-para-methoxyci n nam ate; (4-methoxyphenyl)-2-ethylhexyl ester 2-propenoic acid, 3-(4-methoxyphery- para-A minobenzoic acids (PA BAs) 2-ethoxyethyl ester; 2-ethoxyethyl-4- Trade names: methoxycinnamate AEC Octyl Methoxycinnamate; Escalol Amyl dimethyl FABA 557; Eusolex 2292; Heliosol 3; Empirical formula: Trade names: Jeescreen OMC; Katoscreen OMC; Nec C14H21 NO2 Giv Tan F; Phiasol -

WO 2013/036901 A2 14 March 2013 (14.03.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/036901 A2 14 March 2013 (14.03.2013) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 8/30 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US2012/054376 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 10 September 2012 (10.09.2012) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (26) Publication Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (30) Priority Data: ZM, ZW. 61/532,701 9 September 201 1 (09.09.201 1) US (84) Designated States (unless otherwise indicated, for every (71) Applicant (for all designated States except US): UNIVER¬ kind of regional protection available): ARIPO (BW, GH, SITY OF FLORIDA RESEARCH FOUNDATION, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, INC. -

Les Filtres UV Dans Les Cosmétiques : Une Présence Obligatoire ?
UNIVERSITÉ DE NANTES UFR SCIENCES PHARMACEUTIQUES ET BIOLOGIQUES ____________________________________________________________________________ ANNÉE 2015 N° THÈSE pour le DIPLÔME D’ÉTAT DE DOCTEUR EN PHARMACIE par Anouk POCHAT Présentée et soutenue publiquement le 14 décembre 2015 Les filtres UV dans les cosmétiques : une présence obligatoire ? Président : Mme Laurence Coiffard, Professeur des universités, Laboratoire de Pharmacie industrielle et Cosmétologie Membres du jury : Directeur de thèse : Mme Céline Couteau, Maître de conférences, HDR, Laboratoire de Pharmacie industrielle et Cosmétologie Mme Françoise PEIGNE , Maitre de conférences à la retraite Page 1 Remerciements A mon président de jury, Professeur à la faculté des sciences pharmaceutiques de Nantes J’exprime mes profonds remerciements à Mme Coiffard, pour m’avoir fait l’honneur de présider mon jury de thèse. A mon directeur de thèse, Maître de conférences à la faculté de Pharmacie de Nantes Je remercie Mme Couteau pour m’avoir conseillée et guidée tout au long de mon travail. A Madame Françoise PEIGNE, Docteur en Pharmacie, Je remercie Mme Peigné d’avoir accepté d’assister à ma soutenance. A ma mère, Je te remercie de m’avoir soutenue et encouragée tout au long de mes études. A mon conjoint, Je te remercie pour ta patience, ton écoute et ton soutien. A mes frères, Je vous remercie pour vos encouragements Page 2 I.Introduction Une exposition prolongée aux UVA et aux UVB peut avoir de graves conséquences sur la santé comme, par exemple, la survenue de cancers cutanés (Aubin F., 2001). Les filtres UV permettent d’assurer une protection dans les domaines UVA et/ou UVB. On en trouve dans les produits de protection solaire que le public utilise ponctuellement lors des expositions prolongées au soleil. -

OSEQUE ZSOLE DUAL SUN BLOCK- Octinoxate Avobenzone Bisoctrizole Spray SONGHAK CO., LTD
OSEQUE ZSOLE DUAL SUN BLOCK- octinoxate avobenzone bisoctrizole spray SONGHAK CO., LTD. ---------- Active ingredients: Octyl Methoxycinnamate 7.5%, Ethylhexyl Salicylate 5%, Avobenzone 2%, Bisoctrizole 0.5% Purpose: Sunscreen Inactive Ingredients: Water, Alcohol Denat, Butylene Glycol, Glycerin, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Niacinamide, Isononyl Isononanoate, Glacier Water, Butyloctyl Salicylate, Xanthan Gum, Decyl Glucoside, Disodium EDTA, Methylparaben, Propylparaben, Fragrance Do not use: on wounds, rashes, dermatitis or damaged skin Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately Stop use: Please stop using this product and contact a dermatologist 1. If allergic reaction or irritation occurs 2. If direct sunlight affects the area as above When using this product: Do not use other than directed Warnings: For external use only. Not to be swallowed. Avoid contact with eyes. Discontinue use if signs of irritation or rash appear Uses: Spray or apply liberally over the face and body 15 minutes prior to sun exposure. Always ensure total coverage of all sun exposed areas. Re-apply every 1-2 hours and always after swimming. For spray type, shake it well before use and spray it evenly at 20cm-30cm apart from face or wherever needed. For spray type - shake it well before use For lotion type - turn a cap to use Storage: Keep in a dry or room temperature area OSEQUE ZSOLE DUAL SUN BLOCK octinoxate avobenzone bisoctrizole spray Product