Annex XV Report
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Polycyclic Aromatic Hydrocarbon Structure Index
NIST Special Publication 922 Polycyclic Aromatic Hydrocarbon Structure Index Lane C. Sander and Stephen A. Wise Chemical Science and Technology Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899-0001 December 1997 revised August 2020 U.S. Department of Commerce William M. Daley, Secretary Technology Administration Gary R. Bachula, Acting Under Secretary for Technology National Institute of Standards and Technology Raymond G. Kammer, Director Polycyclic Aromatic Hydrocarbon Structure Index Lane C. Sander and Stephen A. Wise Chemical Science and Technology Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 This tabulation is presented as an aid in the identification of the chemical structures of polycyclic aromatic hydrocarbons (PAHs). The Structure Index consists of two parts: (1) a cross index of named PAHs listed in alphabetical order, and (2) chemical structures including ring numbering, name(s), Chemical Abstract Service (CAS) Registry numbers, chemical formulas, molecular weights, and length-to-breadth ratios (L/B) and shape descriptors of PAHs listed in order of increasing molecular weight. Where possible, synonyms (including those employing alternate and/or obsolete naming conventions) have been included. Synonyms used in the Structure Index were compiled from a variety of sources including “Polynuclear Aromatic Hydrocarbons Nomenclature Guide,” by Loening, et al. [1], “Analytical Chemistry of Polycyclic Aromatic Compounds,” by Lee et al. [2], “Calculated Molecular Properties of Polycyclic Aromatic Hydrocarbons,” by Hites and Simonsick [3], “Handbook of Polycyclic Hydrocarbons,” by J. R. Dias [4], “The Ring Index,” by Patterson and Capell [5], “CAS 12th Collective Index,” [6] and “Aldrich Structure Index” [7]. In this publication the IUPAC preferred name is shown in large or bold type. -

Polycyclic Aromatic Hydrocarbons (Pahs)
Polycyclic Aromatic Hydrocarbons (PAHs) Factsheet 4th edition Donata Lerda JRC 66955 - 2011 The mission of the JRC-IRMM is to promote a common and reliable European measurement system in support of EU policies. European Commission Joint Research Centre Institute for Reference Materials and Measurements Contact information Address: Retiewseweg 111, 2440 Geel, Belgium E-mail: [email protected] Tel.: +32 (0)14 571 826 Fax: +32 (0)14 571 783 http://irmm.jrc.ec.europa.eu/ http://www.jrc.ec.europa.eu/ Legal Notice Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of this publication. Europe Direct is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server http://europa.eu/ JRC 66955 © European Union, 2011 Reproduction is authorised provided the source is acknowledged Printed in Belgium Table of contents Chemical structure of PAHs................................................................................................................................. 1 PAHs included in EU legislation.......................................................................................................................... 6 Toxicity of PAHs included in EPA and EU -

Health Risks of Structural Firefighters from Exposure to Polycyclic
International Journal of Environmental Research and Public Health Systematic Review Health Risks of Structural Firefighters from Exposure to Polycyclic Aromatic Hydrocarbons: A Systematic Review and Meta-Analysis Jooyeon Hwang 1,* , Chao Xu 2 , Robert J. Agnew 3 , Shari Clifton 4 and Tara R. Malone 4 1 Department of Occupational and Environmental Health, Hudson College of Public Health, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA 2 Department of Biostatistics and Epidemiology, Hudson College of Public Health, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; [email protected] 3 Fire Protection & Safety Engineering Technology Program, College of Engineering, Architecture and Technology, Oklahoma State University, Stillwater, OK 74078, USA; [email protected] 4 Department of Health Sciences Library and Information Management, Graduate College, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; [email protected] (S.C.); [email protected] (T.R.M.) * Correspondence: [email protected]; Tel.: +1-405-271-2070 (ext. 40415) Abstract: Firefighters have an elevated risk of cancer, which is suspected to be caused by occupational and environmental exposure to fire smoke. Among many substances from fire smoke contaminants, one potential source of toxic exposure is polycyclic aromatic hydrocarbons (PAH). The goal of this paper is to identify the association between PAH exposure levels and contributing risk factors to Citation: Hwang, J.; Xu, C.; Agnew, derive best estimates of the effects of exposure on structural firefighters’ working environment in R.J.; Clifton, S.; Malone, T.R. Health fire. We surveyed four databases (Embase, Medline, Scopus, and Web of Science) for this systematic Risks of Structural Firefighters from literature review. -

Ocular Effects from Burn Pits in Iraq, Afghanistan, and the Horn of Africa
Ocular effects from Burn Pits in Iraq, Afghanistan, and the Horn of Africa **NOTICE: this is not official VA or C&P released information, this is just information for you to use in assessing patients with exposure to burn pits compiled by Makesha Sink, OD*** Information on burn pits: - Large burn pits have been used throughout the operations in Iraq and Afghanistan to dispose of nearly all forms of waste. It is estimated that such pits, some nearly as large as 20 acres, are or have been located at every military forward operating base (FOB). The pit at Joint Base Balad, also known as Logistic Support Area (LSA) Anaconda, has received the most attention. The burned waste products include, but are not limited to: plastics, metal/aluminum cans, rubber, chemicals (such as, paints, solvents), petroleum and lubricant products, munitions and other unexploded ordnance, wood waste, medical and human waste, and incomplete combustion by- products. Jet fuel (JP-8) is used as the accelerant. The pits do not effectively burn the volume of waste generated, and smoke from the burn pit blows over bases and into living areas. - DoD has performed air sampling at Joint Base Balad, Iraq and Camp Lemonier, Djibouti. Subsequently, DoD has indicated that most of the air samples have not shown individual chemicals that exceed military exposure guidelines (MEG). Nonetheless, DoD further concluded that the confidence level in their risk estimates is low to medium due to lack of specific exposure information, other routes/sources of environmental hazards not identified; and uncertainty regarding the synergistic impact of multiple chemicals present, particularly those affecting the same body organs/systems. -

Fluoranthene
) , "- United States Office of Water EPA 440/5-80-049 Environmental Protection Regulations and Standards October 1980 Agency Criteria and Standards Division Washington DC 20460 aEPA Ambient Water Quality Criteria for Fluoranthene ... AMBIENT WATER QUALITY CRITERIA FOR FLUORANTHENE Prepared By U.S. ENVIRONMENTAL PROTECTION AGENCY Office of Water Regulations and Standards Criteria and Standards Division Washington, D.C. Office of Research and Development Environmental Criteria and Assessment Office Cincinnati, Ohio Carcinogen Assessment Group Washington, D.C. Environmental Research Laboratories Corvalis, Oregon Duluth, Minnesota Gulf Breeze, Florida Narragansett, Rhode Island - -J i DISCLAIMER This report has been reviewed by the Environmental Criteria and Assessment Office, U.S. Environmental Protection Agency, and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. AVAILABILITY NOTICE This document is available to the public through the National Technical Information Service, (NTIS), Springfield, Virginia 22161. ii FOREWORD Section 304 (a) (1) of the Clean Water Act of 1977 (P.L. 95-217), requires the Administrator of the Environmental Protection Agency to publish criteria for water quality accurately reflecting the latest scientific knowledge on the kind and extent of all identifiable effects on health and we 1fare wh ich may be expected from the presence of pollutants in any body of water, including ground water. Proposed water quality criteria for the 65 toxic pollutants listed under section 307 (a) (1) of the Cl ean Water Act were deve loped and a not ice of their availability was published for public comment on March 15, 1979 (44 FR 15926), July 25, 1979 (44 FR 43660), and October 1, 1979 (44 FR 56628). -

2. HEALTH EFFECTS 2.1 INTRODUCTION the Primary
PAHs 11 2. HEALTH EFFECTS 2.1 INTRODUCTION The primary purpose of this chapter is to provide public health officials, physicians, toxicologists, and other interested individuals and groups with an overall perspective of the toxicology of polycyclic aromatic hydrocarbons (PAHs). It contains descriptions and evaluations of toxicological studies and epidemiological investigations and provides conclusions, where possible, on the relevance of toxicity and toxicokinetic data to public health. A glossary and list of acronyms, abbreviations, and symbols can be found at the end of this profile. 2.2 DISCUSSION OF HEALTH EFFECTS BY ROUTE OF EXPOSURE To help public health professionals and others address the needs of persons living or working near hazardous waste sites, the information in this section is organized first by route of exposure-inhalation, oral, and dermal; and then by health effect-death, systemic, immunological, neurological, reproductive, developmental, genotoxic, and carcinogenic effects. These data are discussed in terms of three exposure periods-acute (14 days or less), intermediate (15-364 days), and chronic (365 days or more). Levels of significant exposure for each route and duration are presented in tables and illustrated in figures. The points in the figures showing no-observed-adverse-effect levels (NOAELs) or lowest-observed-adverse-effect levels (LOAELs) reflect the actual doses (levels of exposure) used in the studies. LOAELs have been classified into “less serious” or “serious” effects. “Serious” effects are those that evoke failure in a biological system and can lead to morbidity or mortality (e.g., acute respiratory distress or death). “Less serious” effects are those that are not expected to cause significant dysfunction or death, or those whose significance to the organism is not entirely clear. -

Aerobic Degradation of Fluoranthene, Benzo(B)
Environ & m m en u ta le l o B r t i o e t P e f c Tersagh et al., J Pet Environ Biotechnol 2016, 7:4 h o Journal of Petroleum & n l o a l n o r g DOI: 10.4172/2157-7463.1000292 u y o J ISSN: 2157-7463 Environmental Biotechnology Research Article Open Access Aerobic Degradation of Fluoranthene, Benzo(b)Fluoranthene and Benzo(k) Fluoranthene by Aerobic Heterotrophic Bacteria- Cyanobacteria Interaction in Brackish Water of Bodo Creek Ichor Tersagh*, Ebah EE and Azua ET Department of Biological Sciences, University of Agriculture, Makurdi, Nigeria Abstract The study aimed at ascertaining the biodegradability of polyaromatic hydrocarbons in the fate of fluoranthene, benzo(b)fluoranthene and benzo(k)fluoranthene by aerobic heterotrophic bacteria - cyanobacteria interaction in crude oil contaminated brackish water of Bodo creek. Samples of brackish water were spiked with known volume of Bonny Light crude oil and inoculated with aerobic heterotrophic bacteria and cyanobacteria isolated from crude oil contaminated waters of Bodo creek and monitored for 56 days. The PAHs investigated were quantified using GC-MS where as the bacteria and cyanobacteria isolates were identified on the basis of 16S rRNA gene sequence analysis. The initial quantity of fluoranthene on day 0 for the treatments of Aerobic heterotrophic bacteria (A) 0.43, Cyanobacteria (B) 0.061, and a consortium of A+B 0.24, and the Control, (C) 0.26; Benzo(b)fluoranthene had 0.53, 0.31, 0.65 and 0.66 whereas benzo(k)fluoranthene had 0.62, 0.31, 0.56 and 0.69 mg/l respectively. -

Screening and Determination of Polycyclic Aromatic Hydrocarbons
METHOD NUMBER: C-002.01 POSTING DATE: November 1, 2017 POSTING EXPIRATION DATE: November 1, 2023 PROGRAM AREA: Seafood METHOD TITLE: Screening and Determination of Polycyclic Aromatic Hydrocarbons in Seafoods Using QuEChERS-Based Extraction and High-Performance Liquid Chromatography with Fluorescence Detection VALIDATION STATUS: Equivalent to Level 3 Multi-laboratory validation (MLV) AUTHOR(S): Samuel Gratz, Angela Mohrhaus Bryan Gamble, Jill Gracie, David Jackson, John Roetting, Laura Ciolino, Heather McCauley, Gerry Schneider, David Crockett, Douglas Heitkemper, and Fred Fricke (FDA Forensic Chemistry Center) METHOD SUMMARY/SCOPE: Analyte(s): Polycyclic aromatic hydrocarbons (PAH): acenaphthene, anthracene, benzo[a]anthracene, benzo[a]pyrene, benzo[b]fluoranthene, benzo[g,h,i]perylene, benzo[k]fluoranthene, chrysene, dibenzo[a,h]anthracene, fluoranthene, fluorene, indeno[1,2,3-cd]pyrene, naphthalene, phenanthrene, pyrene Matrices: Oysters, shrimp, crabs, and finfish The method provides a procedure to screen for fifteen targeted parent polycyclic aromatic hydrocarbons (PAHs) and provides an estimate of total PAH concentration including alkylated homologs in oysters, shrimp, crabs, and finfish. PAHs are extracted from seafood matrices using a modified QuEChERS sample preparation procedure. The method utilizes High-Performance Liquid Chromatography with Fluorescence Detection (HPLC-FLD) for the determination step. This procedure is applicable to screen a variety of seafood matrices including oysters, shrimp, finfish and crab for the presence of parent PAHs and the common alkylated homologs due to oil contamination. This method was originally developed and validated in response to the 2010 Gulf of Mexico oil spill. REVISION HISTORY: OTHER NOTES: Method was originally posted on November 1, 2017. It was approved for re-posting by the Chemistry Research Coordination Group for 3 years in December 2020. -

Determining Chemical Air Equivalency Using Silicone Personal Monitors
Journal of Exposure Science & Environmental Epidemiology https://doi.org/10.1038/s41370-021-00332-6 ARTICLE Determining chemical air equivalency using silicone personal monitors 1 2 1 Steven G. O’Connell ● Kim A. Anderson ● Marc I. Epstein Received: 17 December 2020 / Revised: 12 April 2021 / Accepted: 15 April 2021 © The Author(s) 2021. This article is published with open access Abstract Background Silicone personal samplers are increasingly being used to measure chemical exposures, but many of these studies do not attempt to calculate environmental concentrations. Objective Using measurements of silicone wristband uptake of organic chemicals from atmospheric exposure, create log Ksa and ke predictive models based on empirical data to help develop air equivalency calculations for both volatile and semi- volatile organic compounds. Methods An atmospheric vapor generator and a custom exposure chamber were used to measure the uptake of organic chemicals into silicone wristbands under simulated indoor conditions. Log Ksa models were evaluated using repeated k-fold 1234567890();,: 1234567890();,: cross-validation. Air equivalency was compared between best-performing models. Results Log Ksa and log ke estimates calculated from uptake data were used to build predictive models from boiling point 2 (BP) and other parameters (all models: R = 0.70–0.94). The log Ksa models were combined with published data and refined to create comprehensive and effective predictive models (R2: 0.95–0.97). Final estimates of air equivalency using novel BP models correlated well over an example dataset (Spearman r = 0.984) across 5-orders of magnitude (<0.05 to >5000 ng/L). Significance Data from silicone samplers can be translated into air equivalent concentrations that better characterize environmental concentrations associated with personal exposures and allow direct comparisons to regulatory levels. -

Polynuclear Aromatic Hydrocarbons in Drinking-Water
WHO/SDE/WSH/03.04/59 English only Polynuclear aromatic hydrocarbons in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality ______________________ Originally published in Guidelines for drinking-water quality, 2nd ed. Addendum to Vol. 2. Health criteria and other supporting information. World Health Organization, Geneva, 1998. © World Health Organization 2003 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications - whether for sale or for noncommercial distribution - should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use Preface One of the primary goals of WHO and its member states is that “all people, whatever their stage of development and their social and economic conditions, have the right to have access to an adequate supply of safe drinking water.” A major WHO function to achieve such goals is the responsibility “to propose .. -
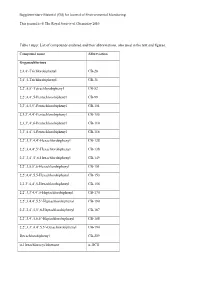
List of Compounds Analysed and Their Abbreviations, Also Used in the Text and Figures
Supplementary Material (ESI) for Journal of Environmental Monitoring This journal is © The Royal Society of Chemistry 2010 Table 1supp: List of compounds analysed and their abbreviations, also used in the text and figures. Compound name Abbreviation Organochlorines 2,4,4’-Trichlorobiphenyl CB-28 2,4’,5-Trichlorobiphenyl CB-31 2,2’,5,5’-Tetrachlorobiphenyl CB-52 2,2’,4,4’,5-Pentachlorobiphenyl CB-99 2,2’,4,5,5’-Pentachlorobiphenyl CB-101 2,3,3’,4,4’-Pentachlorobiphenyl CB-105 2,3,3’,4’,6-Pentachlorobiphenyl CB-110 2,3’,4,4’,5-Pentachlorobiphenyl CB-118 2,2’,3,3’,4,4’-Hexachlorobiphenyl CB-128 2,2’,3,4,4’,5’-Hexachlorobiphenyl CB-138 2,2’,3,4’,5’,6-Hexachlorobiphenyl CB-149 2,2’,3,5,5’,6-Hexachlorobiphenyl CB-151 2,2’,4,4’,5,5-Hexachlorobiphenyl CB-153 2,3,3’,4,4’,5-Hexachlorobiphenyl CB-156 2,2’,3,3’4,4’,5-Heptachlorobiphenyl CB-170 2,2’,3,4,4’,5,5’-Heptachlorobiphenyl CB-180 2,2’,3,4’,5,5’,6-Heptachlorobiphenyl CB-187 2,2’,3,4’,5,6,6’-Heptachlorobiphenyl CB-188 2,2’,3,3’,4,4’,5,5’-Octachlorobiphenyl CB-194 Decachlorobiphenyl CB-209 α-Hexachlorocyclohexane α-HCH Supplementary Material (ESI) for Journal of Environmental Monitoring This journal is © The Royal Society of Chemistry 2010 β-Hexachlorocyclohexane β-HCH γ-Hexachlorocyclohexane γ-HCH Hexachlorobenzene HCB Trans-nonachlor TNC p,p’-dichloro-diphenyl-trichloroethane p,p’-DDT p,p’-dichloro-diphenyl-dichloroethylene p,p’-DDE p,p’-dichloro-diphenyl-dichloroethane p,p’-DDD o,p’-dichloro-diphenyl-trichloroethane o,p’-DDT o,p’-dichloro-diphenyl-dichloroethylene o,p’-DDE PAHs Naphthalene -

Creosote 216
CREOSOTE 216 4. CHEMICAL AND PHYSICAL INFORMATION 4. CHEMICAL AND PHYSICAL INFORMATION 4.1 CHEMICAL IDENTITY The chemical synonyms and identification numbers for wood creosote, coal tar creosote, and coal tar are listed in Tables 4-1 through 4-3. Coal tar pitch is similar in composition to coal tar creosote and is not presented separately. Coal tar pitch volatiles are compounds given off from coal tar pitch when it is heated. The volatile component is not shown separately because it varies with the composition of the pitch. Creosotes and coal tars are complex mixtures of variable composition containing primarily condensed aromatic ring compounds (coal-derived substances) or phenols (wood creosote). Therefore, it is not possible to represent these materials with a single chemical formula and structure. The sources, chemical properties, and composition of coal tar creosote, coal tar pitch, and coal tar justify treating these materials as a whole. Wood creosote is discussed separately because it is different in nature, use, and risk. Information regarding the chemical identity of wood creosote, coal tar creosote, and coal tar is located in Tables 4-1 through 4-3. 4.2 PHYSICAL AND CHEMICAL PROPERTIES Wood creosote, coal tar creosote, coal tar, and coal tar pitch differ from each other with respect to their composition. Descriptions of each mixture are presented below. 4.2.1 Wood Creosote Wood creosotes are derived from beechwood (referred to herein as beechwood creosote) and the resin from leaves of the creosote bush (Larrea, referred to herein as creosote bush resin). Beechwood creosote consists mainly of phenol, cresols, guaiacols, and xylenols.