Electroretinogram Flicker Photometry and Its Applications
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Measuring in Metric Units BEFORE Now WHY? You Used Metric Units
Measuring in Metric Units BEFORE Now WHY? You used metric units. You’ll measure and estimate So you can estimate the mass using metric units. of a bike, as in Ex. 20. Themetric system is a decimal system of measurement. The metric Word Watch system has units for length, mass, and capacity. metric system, p. 80 Length Themeter (m) is the basic unit of length in the metric system. length: meter, millimeter, centimeter, kilometer, Three other metric units of length are themillimeter (mm) , p. 80 centimeter (cm) , andkilometer (km) . mass: gram, milligram, kilogram, p. 81 You can use the following benchmarks to estimate length. capacity: liter, milliliter, kiloliter, p. 82 1 millimeter 1 centimeter 1 meter thickness of width of a large height of the a dime paper clip back of a chair 1 kilometer combined length of 9 football fields EXAMPLE 1 Using Metric Units of Length Estimate the length of the bandage by imagining paper clips laid next to it. Then measure the bandage with a metric ruler to check your estimate. 1 Estimate using paper clips. About 5 large paper clips fit next to the bandage, so it is about 5 centimeters long. ch O at ut! W 2 Measure using a ruler. A typical metric ruler allows you to measure Each centimeter is divided only to the nearest tenth of into tenths, so the bandage cm 12345 a centimeter. is 4.8 centimeters long. 80 Chapter 2 Decimal Operations Mass Mass is the amount of matter that an object has. The gram (g) is the basic metric unit of mass. -

SI and CGS Units in Electromagnetism
SI and CGS Units in Electromagnetism Jim Napolitano January 7, 2010 These notes are meant to accompany the course Electromagnetic Theory for the Spring 2010 term at RPI. The course will use CGS units, as does our textbook Classical Electro- dynamics, 2nd Ed. by Hans Ohanian. Up to this point, however, most students have used the International System of Units (SI, also known as MKSA) for mechanics, electricity and magnetism. (I believe it is easy to argue that CGS is more appropriate for teaching elec- tromagnetism to physics students.) These notes are meant to smooth the transition, and to augment the discussion in Appendix 2 of your textbook. The base units1 for mechanics in SI are the meter, kilogram, and second (i.e. \MKS") whereas in CGS they are the centimeter, gram, and second. Conversion between these base units and all the derived units are quite simply given by an appropriate power of 10. For electromagnetism, SI adds a new base unit, the Ampere (\A"). This leads to a world of complications when converting between SI and CGS. Many of these complications are philosophical, but this note does not discuss such issues. For a good, if a bit flippant, on- line discussion see http://info.ee.surrey.ac.uk/Workshop/advice/coils/unit systems/; for a more scholarly article, see \On Electric and Magnetic Units and Dimensions", by R. T. Birge, The American Physics Teacher, 2(1934)41. Electromagnetism: A Preview Electricity and magnetism are not separate phenomena. They are different manifestations of the same phenomenon, called electromagnetism. One requires the application of special relativity to see how electricity and magnetism are united. -
Metric System.Pdf
METRIC SYSTEM THE METRIC SYSTEM The metric system is much easier. All metric units are related by factors of 10. Nearly the entire world (95%), except the United States, now uses the metric system. Metric is used exclusively in science. Because the metric system uses units related by factors of ten and the types of units (distance, area, volume, mass) are simply-related, performing calculations with the metric system is much easier. METRIC CHART Prefix Symbol Factor Number Factor Word Kilo K 1,000 Thousand Hecto H 100 Hundred Deca Dk 10 Ten Base Unit Meter, gram, liter 1 One Deci D 0.1 Tenth Centi C 0.01 Hundredth Milli M 0.001 Thousandth The metric system has three units or bases. Meter – the basic unit used to measure length Gram – the basic unit used to measure weight Liter – the basic unit used to measure liquid capacity (think 2 Liter cokes!) The United States, Liberia and Burma (countries in black) have stuck with using the Imperial System of measurement. You can think of “the metric system” as a nickname for the International System of Units, or SI. HOW TO REMEMBER THE PREFIXES Kids Kilo Have Hecto Dropped Deca Over base unit (gram, liter, meter) Dead Deci Converting Centi Metrics Milli Large Units – Kilo (1000), Hecto (100), Deca (10) Small Units – Deci (0.1), Centi (0.01), Milli (0.001) Because you are dealing with multiples of ten, you do not have to calculate anything. All you have to do is move the decimal point, but you need to understand what you are doing when you move the decimal point. -
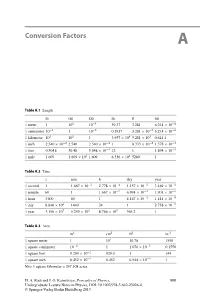
Conversion Factors A
Conversion Factors A Table A.1 Length mcmkmin.ftmi 1 meter 1 102 10−3 39.37 3.281 6.214 × 10−4 1 centimeter 10−2 110−5 0.3937 3.281 × 10−2 6.214 × 10−6 1 kilometer 103 105 13.937 × 104 3.281 × 103 0.621 4 1inch 2.540 × 10−2 2.540 2.540 × 10−5 18.333 × 10−2 1.578 × 10−5 1 foot 0.3048 30.48 3.048 × 10−4 12 1 1.894 × 10−4 1 mile 1 609 1.609 × 105 1.609 6.336 × 104 5280 1 Table A.2 Time s min h day year 1 second 1 1.667 × 10−2 2.778 × 10−4 1.157 × 10−5 3.169 × 10−8 1 minute 60 1 1.667 × 10−2 6.994 × 10−4 1.901 × 10−6 1 hour 3600 60 1 4.167 × 10−2 1.141 × 10−4 1day 8.640 × 104 1440 24 1 2.738 × 10−5 1 year 3.156 × 107 5.259 × 105 8.766 × 103 365.2 1 Table A.3 Area m2 cm2 ft2 in.2 1 square meter 1 104 10.76 1550 1 square centimeter 10−4 11.076 × 10−3 0.1550 1 square foot 9.290 × 10−2 929.0 1 144 1 square inch 6.452 × 10−4 6.452 6.944 × 10−3 1 Note 1 square kilometer = 247.108 acres H. A. Radi and J. O. Rasmussen, Principles of Physics, 999 Undergraduate Lecture Notes in Physics, DOI: 10.1007/978-3-642-23026-4, © Springer-Verlag Berlin Heidelberg 2013 1000 A Conversion Factors Table A.4 Volume m3 cm3 Lft3 in.3 1 cubic meter 1 106 1000 35.51 6.102 × 104 1 cubic centimeter 10−6 11.000 × 10−3 3.531 × 10−5 6.102 × 10−2 1liter 1.000 × 10−3 1000 1 3.531 × 10−2 61.02 1 cubic foot 2.832 × 10−4 1 28.32 1 1728 1 cubic inch 1.639 × 10−4 16.39 1.639 × 10−2 5.787 × 10−4 1 Note 1 U.S. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

Handout – Unit Conversions (Dimensional Analysis)
1 Handout – Unit Conversions (Dimensional Analysis) The Metric System had its beginnings back in 1670 by a mathematician called Gabriel Mouton. The modern version, (since 1960) is correctly called "International System of Units" or "SI" (from the French "Système International"). The metric system has been officially sanctioned for use in the United States since 1866, but it remains the only industrialized country that has not adopted the metric system as its official system of measurement. Many sources also cite Liberia and Burma as the only other countries not to have done so. This section will cover conversions (1) selected units Basic metric units in the metric and American systems, (2) compound or derived measures, and (3) between metric and length meter (m) American systems. Also, (4) applications using conversions will be presented. mass gram (g) The metric system is based on a set of basic units and volume liter (L) prefixes representing powers of 10. time second (s) temperature Celsius (°C) or Kelvin (°K) where C=K-273.15 Prefixes (The units that are in bold are the ones that are most commonly used.) prefix symbol value giga G 1,000,000,000 = 109 ( a billion) mega M 1,000,000 = 106 ( a million) kilo k 1,000 = 103 hecto h 100 = 102 deca da 10 1 1 -1 deci d = 0.1 = 10 10 1 -2 centi c = 0.01 = 10 100 1 -3 milli m = 0.001 = 10 (a thousandth) 1,000 1 -6 micro μ (the Greek letter mu) = 0.000001 = 10 (a millionth) 1,000,000 1 -9 nano n = 0.000000001 = 10 (a billionth) 1,000,000,000 2 To get a sense of the size of the basic units of meter, gram and liter consider the following examples. -

The Metric System
THE METRIC SYSTEM The metric system is a system for measuring volume, length, and mass. This system is based on groups of ten, and its basic prefixes are as follows: Unit Number Line 1000 100 10 1 0.1 0.01 0.001 Kilo- Hecto- Deca- Unit Deci- Centi- Milli- (K) (h) (D) (d) (c) (m) The units are: volume - liter (l) for example: milliliter (ml) length - meter (m) centimeter (cm) mass - gram (g) kilogram (kg) To convert from one prefix to another (milli- to centi-), find out how many of the smaller prefix are in the larger (how many milligrams in a gram). To do this, look at the unit number line above. There is a difference of a multiple of ten between each prefix. To find out how many milligrams are in one gram, count over on the unit line, multiplying by ten for each step. There are 1000 milligrams in 1 gram. You can use the same trick to go from a larger prefix to a smaller prefix. (But remember, there are always more of the smaller prefix than the larger prefix, so the number you get will be how many “smallers” are in the larger.) To go from kilometers to centimeters, use the same process as above, going the opposite direction. (See graphic representation on next page) There are 100,000 centimeters in 1 kilometer. This can be used to convert from one prefix to another. The problems can be set up so that the unwanted prefix will cancel out, leaving the desired prefix. Example 1: Change 50 cm to m. -

Changing Metric Units
Measurement: 6-5 Changing Metric Units MAIN IDEA Change metric units of The lengths of two objects are shown below. length, capacity, and mass. Length Length Object (millimeters) (centimeters) New Vocabulary paper clip 45 4.5 metric system CD case 144 14.4 meter liter 1. Select three other objects. Find and record the width of all five gram kilogram objects to the nearest millimeter and tenth of a centimeter. 2. Compare the measurements of the objects, and write a rule that Math Online describes how to convert from millimeters to centimeters. glencoe.com 3. Measure the length of your classroom in meters. Make a • Extra Examples conjecture about how to convert this measure to centimeters. • Personal Tutor • Self-Check Quiz Explain. 1–3. See margin. The metric system is a decimal system of measures. The prefixes commonly used in this system are kilo-, centi-, and milli-. Meaning Meaning Prefix In Words In Numbers kilo- thousands 1,000 centi- hundredths 0.01 milli- thousandths 0.001 In the metric system, the base unit of length is the meter (m). Using the prefixes, the names of other units of length are formed. Notice that the prefixes tell you how the units relate to the meter. Unit Symbol Relationship to Meter kilometer km 1 km = 1,000 m 1 m = 0.001 km meter m 1 m = 1 m centimeter cm 1 cm = 0.01 m 1 m = 100 cm millimeter mm 1 mm = 0.001 m 1 m = 1,000 mm The liter (L) is the base unit of capacity, the amount of dry or liquid material an object can hold. -

Metric System • the Basic Unit of Length in the Metric System in the Meter and Is Represented by a Lowercase M
Metric System • The basic unit of length in the metric system in the meter and is represented by a lowercase m. • Mass refers to the amount of matter in an object. The base unit of mass in the metric system is the gram and is represented by g. • Volume is the amount of space an object takes up. The base unit of volume in the metric system in the liter and is represented by L or l. LESSON 1: LENGTH Length - English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer 1 mile B. 1 yard or 1 meter 1.6 kilometers C. 1 inch or 1 centimeter 1 inch = 2.54 centimeters 1 yard = 0.9444 meters Metric Units The basic unit of length in the metric system in the meter and is represented by a lowercase m. Metric Units 1 Kilometer (km) = 1000 meters 1 Meter = 100 Centimeters (cm) 1 Meter = 1000 Millimeters (mm) Which is larger? A. 1 meter or 105 centimeters C. 12 centimeters or 102 millimeters B. 4 kilometers or 4400 meters D. 1200 millimeters or 1 meter Measuring Length How many millimeters are in 1 centimeter? 1 centimeter = 10 millimeters What is the length of the line in centimeters? _______cm What is the length of the line in millimeters? _______mm What is the length of the line to the nearest centimeter? ________cm HINT: Round to the nearest centimeter – no decimals. LESSON 2: MASS Mass - English vs. Metric Units Which is larger? 1. 1 Pound or 100 Grams 1 pound = 453.6 grams 2. -

Units of Measure
Units of Measure Units marked with asterisks are base, derived, or supplementary units of the Systeme International. Unit Abbreviation abampere - spell out abohm - spell out abvolt - spell out amagat - spell out *ampere - A ampere hour - A h ampere turns per meter - At/m angstrom - Å arc minute - arc min astronomical unit - AU atmosphere - atm atmosphere, standard - As atomic mass unit - u atomic parts per million - at. ppm atomic percent - at. % atomic time unit - atu atomic unit- a.u. attofarad - aF bar - spell out bark - spell out barn - b barye - spell out biot - Bi bit or bits - spell out blobs per hundred microns - blobs/(100 um) bohr - spell out British thermal unit - Btu bytes - spell out calorie - cal *candela - cd candelas per square meter - cd/m2 candlepower - cp centimeter - cm centipoise - cP *coulomb - C counts per minute - counts/min, cpm counts per second - counts/s cubic centimeter - cm3, (cc not rec.) curie - Ci cycle - spell out, c cycles per second - cps, c/s day d, - or spell out debye - D decibel - dB, dBm degree - [ring], deg degrees - Baumé [ring]B degrees - Celsius (centigrade) [ring]C degrees - Fahrenheit [ring]F degrees - Kelvin K disintegrations per minute - dis/min disintegrations per minute per microgram - dis/min ug disintegrations per second - dis/s dyne - dyn electromagnetic unit - emu electron barn - eb electrons per atom - e/at. electrons per cubic centimeter - e/cm3, e/cc, e cm-3 electron unit - e.u. electron volt - eV electrostatic unit - esu entropy unit - eu erg - spell out *farad - F femtofarad - fF femtometer - fm fermi - F fissions per minute - fpm foot - ft foot-candle - fc foot-lambert - fL foot-pound - ft lb formula units - f.u. -

UNITS of WEIGHT and MEASURE International (Metric) and U.S
I \ ___^am UNITS OF WEIGHT AND MEASURE International (Metric) and U.S. Customary Definitions and Tables of Equivalents ivit I crv¥Hi\u M I I I Arm 'K^ he I I ^Nfck. r a law I I mmm I m mmJr \mw I mum lARE-ACRt STANDARDS U.S. DEPARTMENT OF COMMERCE / NATIONAL BUREAU OF Miscellaneous Publication 286 : THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards 1 provides measurement and technical information services essential to the efficiency and effectiveness of the work of the Nation's scientists and engineers. The Bureau serves also as a focal point in the Federal Government for assur- ing maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To accomplish this mission, the Bureau is organized into three institutes covering broad program areas of research and services: THE INSTITUTE FOR BASIC STANDARDS . provides the central basis within the United States for a complete and consistent system of physical measurements, coor- dinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. This Institute comprises a series of divisions, each serving a classical subject matter area: —Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic Phys- ics—Physical Chemistry—Radiation Physics—Laboratory Astrophysics 2—Radio Standards Laboratory, 2 which includes Radio Standards Physics and Radio Standards Engineering—Office of Standard Reference Data. THE INSTITUTE FOR MATERIALS RESEARCH . conducts materials research and provides associated materials services including mainly reference materials and data on the properties of materials.