Alkaloids from Pandanus Amaryllifolius Collected from Marikina, Philippines
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Threatened Endemic Plants of Palau
THREA TENED ENDEMIC PLANTS OF PALAU BIODI VERSITY CONSERVATION LESSONS LEARNED TECHNICAL SERIES 19 BIODIVERSITY CONSERVATION LESSONS LEARNED TECHNICAL SERIES 19 Threatened Endemic Plants of Palau Biodiversity Conservation Lessons Learned Technical Series is published by: Critical Ecosystem Partnership Fund (CEPF) and Conservation International Pacific Islands Program (CI-Pacific) PO Box 2035, Apia, Samoa T: + 685 21593 E: [email protected] W: www.conservation.org The Critical Ecosystem Partnership Fund is a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the MacArthur Foundation and the World Bank. A fundamental goal is to ensure civil society is engaged in biodiversity conservation. Conservation International Pacific Islands Program. 2013. Biodiversity Conservation Lessons Learned Technical Series 19: Threatened Endemic Plants of Palau. Conservation International, Apia, Samoa Authors: Craig Costion, James Cook University, Australia Design/Production: Joanne Aitken, The Little Design Company, www.thelittledesigncompany.com Photo credits: Craig Costion (unless cited otherwise) Cover photograph: Parkia flowers. © Craig Costion Series Editors: Leilani Duffy, Conservation International Pacific Islands Program Conservation International is a private, non-profit organization exempt from federal income tax under section 501c(3) of the Internal Revenue Code. OUR MISSION Building upon a strong foundation of science, partnership and field demonstration, -

Strengthening of Floristic Diversity in the KFRI Sub Centre Campus Through Planting and Weed Management
KFRI RESEARCH REPORT NO. 443 ISSN 0970-8103 Strengthening of floristic diversity in the KFRI Sub Centre campus through planting and weed management U.M. Chandrashekara Forest Ecology Division Kerala Forest Research Institute (An Institution of Kerala State Council for Science, Technology and Environment) Peechi, Thrissur, Kerala. September,2012 Abstract of Project Proposal Code KFRI 506/2006 Title Strengthening of floristic diversity in the KFRI Sub Centre campus through planting and weed management Objectives 1. To revise the flora of KFRI Sub Centre Campus 2. To adopt water and soil conservation methods for providing suitable habitats for the growth and establishment of seedlings/ propagules Project period April 2006- March 2012 Funded by KFRI Plan Grant Scientific personnel U.M. Chandrashekara CONTENTS ABSTRACT 1 INTRODUCTION 2 MATERIALS AND METHOD 3 RESULTS AND DISCUSSION 3 Floristic study 3 Soil and water management 50 Protection 51 CONCLUSION 52 ACKNOWLEDGEMENTS 53 REFERENCES 53 ABSTRACT A taxonomic survey was carried out to assess the diversity of angiosperm taxa in the campus of Kerala Forest Research Sub Centre at Nilambur. The data were collected during April 2006 to March 2012. A total of 1643 taxa belonging to 152 families were recorded in which 1452 taxa represented species (sub species and natural varities included) and the rest represented cultivars and hybrids. Orchidaceae, Euphorbiaceae and Acanthaceae were families having highest number of taxa, 131, 92 and 71 respectively. Increasing anthropogenic influences on the environment, especially urbanization, have caused negative changes in natural ecosystems in and around Nilambur. In this context, the KFRI Sub Centre campus is an important green campus with its floral richness. -

Int. J. Biosci. 2017
Int. J. Biosci. 2017 International Journal of Biosciences | IJB | ISSN: 2220-6655 (Print), 2222-5234 (Online) http://www.innspub.net Vol. 10, No. 3, p. 80-91, 2017 RESEARCH PAPER OPEN ACCESS Morphological Characterization of Four Pandanus Species of Different Genetic Background Soliman Turki Almutaire1, Magdi Ali Ahmed Mousa*1,2, Ahmed Abdullah Said Bakhashwain1, Ahmed Ibrahim AlQubiee1 1Department of Arid Land Agriculture Faculty of Metrology, Environment and Arid Land Agriculture, University of King Abdulaziz, Jeddah, Saudi Arabia 1Department of Vegetables, Faculty of Agriculture, University of Assiut, Assiut, Egypt Key words: Kewda, Pandanus spp, Morphological characterization, Pandanus odoratissimus http://dx.doi.org/10.12692/ijb/10.3.80-91 Article published on March 12, 2017 Abstract The present study was carried out in the lab. of plant tissue culture at King Abdulaziz University to investigate morphological characterization of four Pandanus species of different genetic background. The used Pandanus species were Pandanus odoratissimus naturally grown in different regions of Saudi Arabia and Pandanus dubius, Pandanus tectorius var 'Variegata' and Pandanus tectorius var ‘Utilis’ collected from the agriculture market of Jeddah, Saudi Arabia. About 15 seedling of each species were organized in Randomized Complete Block (CRD) using 5 replicates. Morphological characterization was carried out using leaves of different ages of Pandanus species. The results showed that Pandanus tectorius var 'Variegata' revealed highest leaf breadth, leaf thickness and leaf contents of P (%). Pandanus odoratissimus illustrated highest Ch a and Ch b contents (mg/100g). Pandanus dubius recorded highest leaf length (cm). leaves of medium ages of Pandanus (leaves found in the meddle layers of plant crown) revealed highest values of leaf length (cm), leaf breadth (cm), leaf thickness (mm), leaf fresh and dry weight (g), and Ch a and Ch b (mg/100g). -

Multipurpose Trees for Agroforestry in the Pacific Islands
For Educators, Gardeners, Farmers, Foresters, and Landscapers Agroforestry Guides for Pacific Islands “Well-researched, concise, user-friendly...an invaluable practical resource for those working to conserve and expand the use of trees in agricultural systems.” —APANews, The Asia-Pacific Agroforestry Newsletter FAO Regional Office, Bangkok, Thailand “A significant contribution to public education, advancing the cause of integrated agriculture and forestry...a resource of lasting value.” —The Permaculture Activist, North Carolina “A most excellent handbook...a wonderful resource.” —Developing Countries Farm Radio Network, Toronto, Canada “Eloquently makes a case for reintroducing and emphasizing trees in our island agriculture.” —Dr. Bill Raynor, Program Director, The Nature Conservancy, Pohnpei, Federated States of Micronesia “Provides a real clearinghouse on traditional and modern agroforestry not only for Pacific Islands, also very useful for other regions.” —ILEIA Newsletter for Low External Input and Sustainable Agriculture, The Netherlands Purchase the book at http://www.agroforestry.net/afg/ Agroforestry Guides for Pacific Islands edited by Craig R. Elevitch and Kim M. Wilkinson Price: $24.95 (plus shipping) Availability: Usually ships within one business day. Paperback - 240 pages, illustrated and fully indexed Release date: September, 2000 ISBN: 0970254407 Publisher: Permanent Agriculture Resources, P.O. Box 428, Holualoa, HI, 96725, USA. Tel: 808-324-4427, Fax: 808-324-4129, email: [email protected] Agroforestry Guides for Pacific Islands #2 Multipurpose Trees for Agroforestry in the Pacific Islands by Randolph R. Thaman, Craig R. Elevitch, and Kim M. Wilkinson www.agroforestry.net Multipurpose Trees for Agroforestry in the Pacific Islands Abstract: The protection and planting of trees in agroforestry systems can serve as an important, locally achievable, and cost-effective step in sustainable development in the Pacific Islands. -

Field Instructions for the Periodic Inventory of The
FIELD INSTRUCTIONS FOR THE PERIODIC INVENTORY OF THE COMMONWEALTH OF THE NORTHERN MARIANA ISLANDS 2015 FOREST INVENTORY AND ANALYSIS RESOURCE MONITORING AND ASSESSMENT PROGRAM PACIFIC NORTHWEST RESEARCH STATION USDA FOREST SERVICE THIS MANUAL IS BASED ON: FOREST INVENTORY AND ANALYSIS NATIONAL CORE FIELD GUIDE VOLUME I: FIELD DATA COLLECTION PROCEDURES VERSION 6.1 Cover image by Gretchen Bracher pg.I Table of Contents CHAPTER 1 INTRODUCTION . 15 SECTION 1.1 ORGANIZATION OF THIS MANUAL. 15 SECTION 1.2 THE INVENTORY. 16 SECTION 1.3 PRODUCTS . 16 SECTION 1.4 UNITS OF MEASURE . 16 SECTION 1.5 PLOT DESIGN GENERAL DESCRIPTION . 16 SUBSECTION 1.5.1 PLOT LAYOUT . .17 SUBSECTION 1.5.2 DATA ARE COLLECTED ON PLOTS AT THE FOLLOWING LEVELS .17 SECTION 1.6 QUALITY ASSURANCE/QUALITY CONTROL . 18 SUBSECTION 1.6.1 GENERAL DESCRIPTION . .18 SECTION 1.7 SAFETY . 18 SUBSECTION 1.7.1 SAFETY IN THE WOODS. .18 SUBSECTION 1.7.2 SAFETY ON THE ROAD . .19 SUBSECTION 1.7.3 WHAT TO DO IF INJURED. .19 CHAPTER 2 LOCATING THE PLOT . 21 SECTION 2.1 LOCATING AN ESTABLISHED PLOT . 21 SUBSECTION 2.1.1 NAVIGATING WITH PHOTOGRAPHY. .21 SUBSECTION 2.1.2 NAVIGATING WITH GPS . .21 SUBSECTION 2.1.3 NAVIGATING WITH REFERENCE POINT (RP) DATA . .22 SUBSECTION 2.1.4 REVERSE REFERENCE POINT (RP) METHOD . .22 SECTION 2.2 ESTABLISHED PLOT ISSUES . 22 SUBSECTION 2.2.1 DIFFICULTY FINDING ESTABLISHED PLOTS. 22 SUBSECTION 2.2.2 INCORRECTLY INSTALLED PLOT . .23 SUBSECTION 2.2.3 INCORRECTLY INSTALLED SUBPLOT OR MICROPLOT. .23 SUBSECTION 2.2.4 PC STAKE OR SUBPLOT/MICROPLOT PIN MISSING OR MOVED . -

EACPT 2015 Madrid, Spain
Jurnal Intelek (2016) Vol 11(1): 1-6 ISSN 2231-7716 © PJIM&A, UiTM Perlis The Nomenclature of Pandanus Alkaloids Ibtisam Abdul Wahab, Hannis Fadzillah Mohsin and Ummu Amirah Armayni Department of Pharmacology & Chemistry, Faculty of Pharmacy, Universiti Teknologi MARA, 42300 Puncak Alam, Selangor Darul Ehsan Email: [email protected] Abstract From the literature, only one percent of the total Pandanus species (Pandanaceae family) were analysed in the studies related to their secondary metabolites. Scientific publications offered neither a recent record of all Pandanus alkaloids, nor the suggestion of any classification of these biomolecules. Therefore, this paper is aimed to propose the unprecedented nomenclature of Pandanus alkaloids, following the inventory of their 26 natural alkaloids. From the examination, these specific compounds have a basic C9- N-C9 molecular framework. For an example, in the arrangement of carbon atoms of Pandanamine, the first carbon (C-1) is counted at the heteroatom of oxygen and the second carbon is attached with the ketone group. The C-1 to C-9 was assigned with one lactone skeleton. In the meantime, C-11 to C-19 showed the second lactone moiety. Furthermore, this nomenclature is determined based on its amine group. Pandanamine, being a symmetrical secondary amine, is hypothetically a biogenetic precursor of the Pandanus alkaloids. It has a straight chain of anime group that undergoes intramolecular cyclisation to form heterocyclic amines such as pyrrolidine (five-membered ring), piperidine (six-membered ring) and indolizidine (five- and six-membered rings). Thus, the compound that consists of piperidine alkaloid was named as pandamarilactone. Meanwhile, the molecules having pyrrolidine alkaloid was referred as pandamarilactonine. -

Botanical Survey of the War in the Pacific National Historical Park Guam, Mariana Islands
PACIFIC COOPERATIVE STUDIES UNIT UNIVERSITY OF HAWAI`I AT MĀNOA Dr. David C. Duffy, Unit Leader Department of Botany 3190 Maile Way, St. John #408 Honolulu, Hawai’i 96822 Technical Report 161 Botanical survey of the War in the Pacific National Historical Park Guam, Mariana Islands July 2008 Joan M. Yoshioka 1 1 Pacific Cooperative Studies Unit (University of Hawai`i at Mānoa), NPS Inventory and Monitoring Program, Pacific Island Network, PO Box 52, Hawai`i National Park, HI 96718 PCSU is a cooperative program between the University of Hawai`i and U.S. National Park Service, Cooperative Ecological Studies Unit. Organization Contact Information: Inventory and Monitoring Program, Pacific Island Network, PO Box 52, Hawaii National Park, HI 96718, phone: 808-985-6183, fax: 808-985-6111 Recommended Citation: Yoshioka, J. M. 2008. Botanical survey of the War in the Pacific National Historical Park Guam, Mariana Islands. Pacific Cooperative Studies Unit Technical Report 161, University of Hawai`i at Manoa, Department of Botany, Honolulu, HI. Key words: Vegetation types, Vegetation management, Alien species, Endemic species, Checklist, Ferns, Flowering plants Place key words: War in the Pacific National Historical Park, Guam Editor: Clifford W. Morden, PCSU Deputy Director (Mail to: mailto:[email protected]) i Table of Contents List of Tables......................................................................................................iii List of Figures ....................................................................................................iii -

Perennial Edible Fruits of the Tropics: an and Taxonomists Throughout the World Who Have Left Inventory
United States Department of Agriculture Perennial Edible Fruits Agricultural Research Service of the Tropics Agriculture Handbook No. 642 An Inventory t Abstract Acknowledgments Martin, Franklin W., Carl W. Cannpbell, Ruth M. Puberté. We owe first thanks to the botanists, horticulturists 1987 Perennial Edible Fruits of the Tropics: An and taxonomists throughout the world who have left Inventory. U.S. Department of Agriculture, written records of the fruits they encountered. Agriculture Handbook No. 642, 252 p., illus. Second, we thank Richard A. Hamilton, who read and The edible fruits of the Tropics are nnany in number, criticized the major part of the manuscript. His help varied in form, and irregular in distribution. They can be was invaluable. categorized as major or minor. Only about 300 Tropical fruits can be considered great. These are outstanding We also thank the many individuals who read, criti- in one or more of the following: Size, beauty, flavor, and cized, or contributed to various parts of the book. In nutritional value. In contrast are the more than 3,000 alphabetical order, they are Susan Abraham (Indian fruits that can be considered minor, limited severely by fruits), Herbert Barrett (citrus fruits), Jose Calzada one or more defects, such as very small size, poor taste Benza (fruits of Peru), Clarkson (South African fruits), or appeal, limited adaptability, or limited distribution. William 0. Cooper (citrus fruits), Derek Cormack The major fruits are not all well known. Some excellent (arrangements for review in Africa), Milton de Albu- fruits which rival the commercialized greatest are still querque (Brazilian fruits), Enriquito D. -

Department of the Interior
Vol. 80 Thursday, No. 190 October 1, 2015 Part IV Department of the Interior Fish and Wildlife Service 50 CFR Part 17 Endangered and Threatened Wildlife and Plants; Endangered Status for 16 Species and Threatened Status for 7 Species in Micronesia; Final Rule VerDate Sep<11>2014 21:53 Sep 30, 2015 Jkt 238001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\01OCR3.SGM 01OCR3 mstockstill on DSK4VPTVN1PROD with RULES3 59424 Federal Register / Vol. 80, No. 190 / Thursday, October 1, 2015 / Rules and Regulations DEPARTMENT OF THE INTERIOR (TDD) may call the Federal Information of the physical or biological features Relay Service (FIRS) at 800–877–8339. essential to the species’ conservation. Fish and Wildlife Service SUPPLEMENTARY INFORMATION: Information regarding the life functions and habitats associated with these life 50 CFR Part 17 Executive Summary functions is complex, and informative Why we need to publish a rule. Under data are largely lacking for the 23 [Docket No. FWS–R1–ES–2014–0038; the Endangered Species Act of 1973, as Mariana Islands species. A careful 4500030113] amended (Act or ESA), a species may assessment of the areas that may have RIN 1018–BA13 warrant protection through listing if it is the physical or biological features endangered or threatened throughout all essential for the conservation of the Endangered and Threatened Wildlife or a significant portion of its range. species and that may require special and Plants; Endangered Status for 16 Listing a species as an endangered or management considerations or Species and Threatened Status for 7 threatened species can only be protections, and thus qualify for Species in Micronesia completed by issuing a rule. -

A Geographical Checklist of the Micronesian Monocotyledonae
A Geographical Checklist of the Micronesian Monocotyledonae F. R. FOSBERG,MARIE-HELENE SACHET and ROYCE OLIVER B01a11yDepar1me111. Narional M11se11m ofNa111ra/Hisrory, Smi1hso11ia11ills1i1111io11, Washi11g1011, D.C . 20560 Abstract. The monocotyledonous plants known to us as occurring or reported to have occurred in Micronesia are listed systematically by families and alphabetically under the families. They belong to 272 genera (in a broad sense) . Of the 677 species and varieties included, 367 are considered indigenous, 308 exotic (indicated by an asterisk). Of the indigenous ones, 166 are endemic in Mi- cronesia, so far as we know. The other 204 extend to other lndo-Pacific islands or countries border- ing the lndo-Pacilic region. The strand and coastal plants are mostly widespread, some are pan- tropical. Of the endemics, 135 are restricted to the Caroline Islands, 19 to the Marianas, 11 to both groups, and one to Wake and the northern Marshalls. There are no endemics, (with the possible exception of the poorly known Crim1m bakeri) in the central and southern Marshalls, the Gilberts, or the other isolated islands . The affinities of the species are mostly lndo-Malaysian and New Gui- nean-Melanesian, a few are Australian . For each species and smaller taxon the known distribution by islands is given, as well as basionyms, synonyms, misapplications or misidentilications that have appeared in the Micronesian literature known to us. Introduction The third and last part of our Geographical Checklist of Micronesian Vascular Plants includes all taxa of Monocotyledonae known to us from Micronesia. Earlier parts covered the dicotyledons, and pteridophytes and gymnosperms (Micronesica 15: 41-295, 1979; 18: 23-82, 1982). -
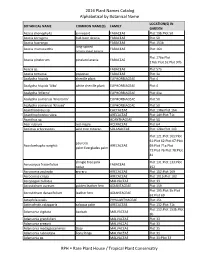
2016 Plant Names Catalog Alphabetical by Botanical Name
2016 Plant Names Catalog Alphabetical by Botanical Name LOCATION(S) IN BOTANICAL NAME COMMON NAME(S) FAMILY GARDEN Acacia choriophylla cinnecord FABACEAE Plot 19b:Plot 50 Acacia cornigera bull-horn Acacia FABACEAE Plot 50 Acacia huarango FABACEAE Plot 153b long-spined Acacia macracantha FABACEAE Plot 164 acacia:steel Acacia Plot 176a:Plot Acacia pinetorum pineland acacia FABACEAE 176b:Plot 3a:Plot 97b Acacia sp. FABACEAE Plot 57a Acacia tortuosa poponax FABACEAE Plot 3a Acalypha hispida chenille plant EUPHORBIACEAE Plot 4 Acalypha hispida 'Alba' white chenille plant EUPHORBIACEAE Plot 4 Acalypha 'Inferno' EUPHORBIACEAE Plot 41a Acalypha siamensis 'Firestorm' EUPHORBIACEAE Plot 50 Acalypha siamensis 'Kilauea' EUPHORBIACEAE Plot 50 Acanthocereus sp. CACTACEAE Plot 138a:Plot 164 Acanthophoenix rubra ARECACEAE Plot 149:Plot 71c Acanthus sp. ACANTHACEAE Plot 50 Acer rubrum red maple ACERACEAE Plot 64 Acnistus arborescens wild tree tobacco SOLANACEAE Plot 128a:Plot 143 Plot 121:Plot 161:Plot 61:Plot 62:Plot 67:Plot paurotis Acoelorrhaphe wrightii ARECACEAE 69:Plot 71a:Plot palm:Everglades palm 72:Plot 76:Plot 78:Plot 81 shingle tree:pink Plot 131:Plot 133:Plot Acrocarpus fraxinifolius FABACEAE cedar 152 Acrocomia aculeata gru-gru ARECACEAE Plot 102:Plot 169 Acrocomia crispa ARECACEAE Plot 101b:Plot 102 Acropogon bullatus MALVACEAE Plot 33 Acrostichum aureum golden leather fern ADIANTACEAE Plot 159 Plot 195:Plot 3b:Plot Acrostichum danaeifolium leather fern ADIANTACEAE 63:Plot 69 Actephila ovalis PHYLLANTHACEAE Plot 151 Actinorhytis calapparia calappa palm ARECACEAE Plot 132:Plot 71c Plot 112:Plot 153b:Plot Adansonia digitata baobab MALVACEAE 3b Adansonia grandidieri MALVACEAE Plot 33 Adansonia gregorii MALVACEAE Plot 33 Adansonia madagascariensis Bozy MALVACEAE Plot 35 Adansonia rubrostipa Fony:Ringy MALVACEAE Plot 31 Adansonia sp. -

Pandanaceae of the Island of Yapen, Papua
Blumea 54, 2009: 255–266 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE doi:10.3767/000651909X476247 Pandanaceae of the island of Yapen, Papua (West New Guinea), Indonesia, with their nomenclature and notes on the rediscovery of Sararanga sinuosa, and several new species and records A.P. Keim1 Key words Abstract Eleven species of Pandanaceae are recorded for Yapen Island, Papua, Indonesia, seven of Pandanus, three of Freycinetia, including two new ones, and the rediscovery of Sararanga sinuosa. Except for the latter all Freycinetia others are new records for the island. New Guinea Pandanaceae Published on 30 October 2009 Pandanus Papua Sararanga Yapen INTRODUCTION and lateral, mostly lateral (4 individuals observed, only 1 with terminal infructescence), non aromatic, ternate or quaternate, Yapen is one of the islands in the Cenderawasih (Geelvink) Bay each c. 15 cm long; peduncle 3–3.5 cm long, yellowish green, in the Indonesian Province of Papua, West New Guinea. The scabrous; bracts 4, bright yellow, unequally, the most inner one island is about 2 400 km², of which approximately 3/4 is still being smaller, thick, fleshy, caducous, each 9.5–10 cm long, covered with lavish lowland tropical rainforest, and an area of c. 5 cm wide, boat-shaped with acuminate apex. Cephalium about 780 km² is protected as the Yapen Tengah Nature Re- sausage-shaped, corky-warted surface, 10–11 cm long, c. 8.5 serve. Despite the magnificent landscape, Yapen compared to cm circumference (2.7–3 cm diam), green, slightly glaucous its neighbouring island Biak has remained little explored. Since white, consisting of numerous berries.