Proceedings-2006.Pdf
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Emissions of Such Gases on a ‘CO2- Equivalent’ Scale
Department of Meteorology Assessing the potential climate impacts of industrial gases Professor Keith Shine FRS | Professor Eleanor Highwood Summary Human activity leads to the emission of many greenhouse gases that differ from carbon dioxide (CO2) in their effects on climate. International climate policy requires the use of an ‘exchange rate’ to place emissions of such gases on a ‘CO2- equivalent’ scale. These ‘exchange rates’ are calculated using ‘climate emission metrics’, which enable quantitative comparisons to be made of the climate impact of the emission of a given gas with respect to CO2 emissions. Background The assessment reports of the Intergovernmental Panel on Climate Change (IPCC) and the World Meteorological Organization / United Nations Environment Programme (WMO/UNEP) Scientific Assessments of Stratospheric Ozone Depletion presented the values of ‘Global Warming Potential’ or GWP. GWP is the metric adopted by the Kyoto Protocol to the United Nations’ Framework Convention on Climate Change (UNFCCC) to allow signatories to report emissions of different greenhouse gases on a CO2-equivalent scale, and is one of a range of possible methods for comparing the climate impact of emissions of different greenhouse gases. How is University of Reading research contributing? Professor Keith Shine played a major role in the international assessments. He led the compilation of databases of an essential input to GWP calculations, namely the so-called ‘radiative efficiency’, or RE, for gases included in the Kyoto Protocol. He and his co-workers within and outside the Department of Meteorology developed and refined methods for calculating RE, using advanced numerical models incorporating new laboratory observations. For many industrial gases, the Reading group has presented the first published RE value, and it has helped resolve instances where results presented in the literature had been in substantive disagreement. -

Metrics for Comparison of Climate Impacts from Well Mixed
1 ACCRI Theme 7 2 3 Metrics for comparison of climate impacts from well mixed greenhouse gases and 4 inhomogeneous forcing such as those from UT/LS ozone, contrails and contrail- 5 cirrus 6 7 Piers Forster & Helen Rogers 8 9 Acknowledgement: The numbers in Table 5 and a many of the ideas are derived from a 10 unpublished manuscript led by Keith Shine that Piers Forster and Helen Rogers were co- 11 author of. 12 13 Executive Summary.......................................................................................................... 2 14 1. Introduction and Background ................................................................................. 5 15 2. Review ........................................................................................................................ 8 16 2.1. Current state of science....................................................................................... 8 17 2.1.1. Air travel – its emissions and its trends ...................................................... 8 18 2.1.2. Aviation’s climate impact......................................................................... 10 19 2.1.3. Review of the RF characteristics and uncertainties of mechanisms ......... 12 20 2.1.3.1. Chemistry of importance to aviation..................................................... 12 21 2.1.3.2. Modelling the impact of aviation.......................................................... 14 22 2.1.4. Regional and timescale issues................................................................... 16 23 2.2. Critical -

Trends and Patterns in the Contributions to Cumulative Radiative Forcing from Different Regions of the World
Trends and patterns in the contributions to cumulative radiative forcing from different regions of the world D. M. Murphya,1 and A. R. Ravishankarab,c,1 aEarth System Research Laboratory, Chemical Sciences Division, National Oceanic and Atmospheric Administration, Boulder, CO 80305; bDepartment of Chemistry, Colorado State University, Fort Collins, CO 80524; and cDepartment of Atmospheric Science, Colorado State University, Fort Collins, CO 80524 Contributed by A. R. Ravishankara, November 12, 2018 (sent for review August 17, 2018; reviewed by John H. Seinfeld and Keith Shine) Different regions of the world have had different historical very short lived, on the order of weeks, so that their radiative patterns of emissions of carbon dioxide, other greenhouse gases, influence is essentially simultaneous with their emission time. and aerosols as well as different land-use changes. One can estimate The relative emissions of GHGs and aerosols have not been the net cumulative contribution by each region to the global mean the same in different regions. One reason is that some regions radiative forcing due to past greenhouse gas emissions, aerosol have had more emissions from industrial activity than other re- precursors, and carbon dioxide from land-use changes. Several gions, and in other regions land-use/land-cover change (LULC) patterns stand out from such calculations. Some regions have had a has been an important driver of emissions. Here we explicitly common historical pattern in which the short-term offsets between deal with only the largest component of the latter––the influence the radiative forcings from carbon dioxide and sulfate aerosols of LULC on CO2. -
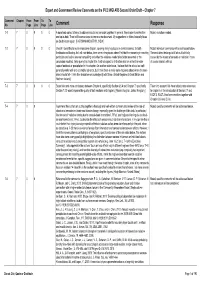
Comment Response 7-1 7 0 0 0 0 a Novel and Correct Attempt to Address Clouds and Aerosols Together
Expert and Government Review Comments on the IPCC WGI AR5 Second Order Draft – Chapter 7 Comment Chapter From From To To No Page Line Page Line Comment Response 7-1 7 0 0 0 0 A novel and correct attempt to address clouds and aerosols together. In general, the chapter is well-written Noted, no action needed. and up-to-date. There still is some scope to improve the document. My suggestions to follow, basically focus on South Asian region [K KRISHNA MOORTHY, INDIA] 7-2 7 0 0 0 0 Overall I found this to be an impressive chapter, covering many key topics in climate science, for both Noted. Individual comments will be addressed below. feedbacks and forcing. As I will note below, there were a few places where I felt that the reasoning in reaching The semi-direct belongs to AFari as it is initially particular conclusions was not compelling and either the evidence needs to be better presented or the caused by the impact of aerosols on radiation. It can conclusion modified. I also query the chapter title. I did not expect to find information on either the water of course interact with aci. vapour feedback or precipitation in this chapter. On another wider issue, I believe that the ari and aci split generally works well and is a helpful advance, but I think there is really some haziness about where the semi- direct should fall - I think this should be acknowledged [Keith Shine, United Kingdom of Great Britain and Northern Ireland] 7-3 7 0 0 0 0 Better links and more consistency between Chapter 6, specifically Section 6.5.4 and Chapter 7, specifically Taken into account. -

Climate Change: Evidence & Causes 2020
Climate Change Evidence & Causes Update 2020 An overview from the Royal Society and the US National Academy of Sciences n summary Foreword CLIMATE CHANGE IS ONE OF THE DEFINING ISSUES OF OUR TIME. It is now more certain than ever, based on many lines of evidence, that humans are changing Earth’s climate. The atmosphere and oceans have warmed, which has been accompanied by sea level rise, a strong decline in Arctic sea ice, and other climate-related changes. The impacts of climate change on people and nature are increasingly apparent. Unprecedented flooding, heat waves, and wildfires have cost billions in damages. Habitats are undergoing rapid shifts in response to changing temperatures and precipitation patterns. The Royal Society and the US National Academy of Sciences, with their similar missions to promote the use of science to benefit society and to inform critical policy debates, produced the original Climate Change: Evidence and Causes in 2014. It was written and reviewed by a UK-US team of leading climate scientists. This new edition, prepared by the same author team, has been updated with the most recent climate data and scientific analyses, all of which reinforce our understanding of human-caused climate change. The evidence is clear. However, due to the nature of science, not every detail is ever totally settled or certain. Nor has every pertinent question yet been answered. Scientific evidence continues to be gathered around the world. Some things have become clearer and new insights have emerged. For example, the period of slower warming during the 2000s and early 2010s has ended with a dramatic jump to warmer temperatures between 2014 and 2015. -

Awards and Prizes 2019
Promoting the understanding and application of meterology for the benefit of all Awards and Prizes 2019 rmets.org theweatherclub.org.uk metlink.org Awards and Prizes 2019 1 ContentsHighlights Page The Mason Gold Medal 4 The Buchan Prize 5 The L F Richardson Prize 6 Message from Professor Liz Bentley – The FitzRoy Prize 8 Chief Executive The Adrian Gill Prize 9 I am delighted to announce our 2019 Award and The Climate Science Communications Award 10 Prize winners. Each year we take the opportunity to recognise people and teams who have made The Society’s Outstanding Service Award 11 exceptional contributions to weather, climate and associated disciplines as worthy recipients of The Gordon Manley Weather Prize 12 our Awards and Prizes. We received some outstanding nominations this year with many The Malcolm Walker Award 13 individuals being recognised internationally for their remarkable work. Honorary Fellow 14 This year we celebrate our 170th Anniversary. Progress made over the last 170 The International Journal of Climatology Award 15 years in technology and our understanding of weather and climate, as well as the huge public interest, would amaze our founding members. The work of The Quarterly Journal Editor’s Award 15 our Award and Prize winners demonstrates and showcases this progress. Recent extreme weather events underline our dependency on reliable and The Geoscience Data Journal Editor’s Award 16 timely information and the importance of dealing with the threat from man- The Atmospheric Science Letters Editor’s Award 16 made climate change. The Society continues to play a key role in supporting the science and profession for the benefit of all and our Awards and Prizes The Quarterly Journal Reviewer’s Certificate 17 are a crucial part of this work. -

The Fastest Wind Powered Vehicle on Earth
Cover:Layout 1 23/9/09 12:16 Page 2 Imperial 34 mattersSummer | 2009 Alumni magazine of Imperial College London including the former Charing Cross and Westminster Medical School, Royal Postgraduate Medical School, St Mary’s Hospital Medical School and Wye College h Natural selection Meet Imperial’s evolutionary biologists The fastest Climate change Sir Brian Hoskins on why we must change wind powered the future Plus all the news from the College vehicle on Earth and alumni groups Cover:Layout 1 23/9/09 12:17 Page 3 Summer 2009 contents//34 18 22 24 news features alumni cover 2 College 10 Faster than the 28 Services The land yacht, called the 4 Business speed of wind 30 UK Greenbird, used Alumnus breaks the world land PETER LYONS by alumnus 5 Engineering speed record for a wind 34 International Richard Jenkins powered vehicle to break the 6 Medicine 38 Catch up world land 14 Charles Darwin and speed record for 7 Natural Sciences his fact of evolution 42 Books a wind powered 8 Arts and sport Where Darwin’s ideas sit 44 In memoriam vehicle sits on Lake Lafroy in 150 years on Australia awaiting world record 9 Felix 45 The bigger picture breaking conditions. 18 It’s not too late Brian Hoskins on climate change 22 The science of flu Discover the workings of the influenza virus 24 The adventurer Alumnus Simon Murray tells all about his impetuous life Imperial Matters is published twice a year by the Office of Alumni and Development and Imperial College Communications. Issue 35 will be published in January 2010. -

Discerning Experts
Discerning Experts Discerning Experts The Practices of Scientific Assessment for Environmental Policy MICHAEL OPPENHEIMER, NAOMI ORESKES, DALE JAMIESON, KEYNYN BRYSSE, JESSICA O’REILLY, MATTHEW SHINDELL, AND MILENA WAZECK University of Chicago Press chicago and london The University of Chicago Press, Chicago 60637 The University of Chicago Press, Ltd., London © 2019 by The University of Chicago All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without written permission, except in the case of brief quotations in critical articles and reviews. For more information, contact the University of Chicago Press, 1427 E. 60th St., Chicago, IL 60637. Published 2019 Printed in the United States of America 28 27 26 25 24 23 22 21 20 19 1 2 3 4 5 isbn- 13: 978- 0- 226- 60196- 0 (cloth) isbn- 13: 978- 0- 226- 60201- 1 (paper) isbn- 13: 978- 0- 226- 60215- 8 (e- book) doi: https://doi.org/10.7208/chicago/9780226602158.001.0001 Library of Congress Cataloging-in-Publication Data Names: Oppenheimer, Michael, author. | Oreskes, Naomi, author. | Jamieson, Dale, author. | Brysse, Keynyn, author. | O’Reilly, Jessica, 1978– author. | Shindell, Matthew, author. | Wazeck, Milena, author. Title: Discerning experts : the practices of scientific assessment for environmental policy / Michael Oppenheimer, Naomi Oreskes, Dale Jamieson, Keynyn Brysse, Jessica O’Reilly, Matthew Shindell, and Milena Wazeck. Description: Chicago ; London : University of Chicago Press, 2019. | Includes bibliographical references and index. Identifiers: lccn 2018021455 | isbn 9780226601960 (cloth : alk. paper) | isbn 9780226602011 (pbk. : alk. paper) | isbn 9780226602158 (e-book) Subjects: lcsh: Environmental sciences—Research—Evaluation. | Environmental sciences—Research—Evaluation—Case studies. -

BC Policy-Relevant Summary Final
A policy-relevant summary of black carbon climate science and appropriate emission control strategies June 2009 The goal of the International Council on Clean Transportation is to dramatically improve the environmental performance and efficiency of cars, trucks, ships, airplanes and the transportation systems that support them with the aim to protect and improve public health, the environment, and quality of life. The organization is guided by a Council of regulators and experts from leading auto markets around the world who participate as individuals based on their experience with air quality and transportation issues. Michael Walsh Dr. Alan Lloyd International Transportation Consultant President United States International Council on Clean Transportation United States Mary Nichols Chairwoman Dan Greenbaum California Air Resources Board President United States Health Effects Institute United States Dr. Adrian Fernandez Bremauntz President Jiro Hanyu National Institute of Ecology President México Japan International Transport Institute Japan Dr. Leonora Rojas Bracho Director General Dagang Tang Research on Urban and Regional Pollution Director National Institute of Ecology Vehicle Emission Control Center México Ministry of Environmental Protection China Dr. Martin Williams Director Dr. Yasuhiro Daisho Atmospheric Quality and Industrial Pollution Programme Professor of Engineering Department of Environment Food, and Rural Affairs Waseda University United Kingdom Japan Dr. Axel Friedrich Dr. Mario Molina Former Director Professor Environment, Transport and Noise Division Department of Chemistry and Biochemistry Federal Environmental Agency University of Cailfornia, San Diego Germany and President Centro Mario Molina México © 2009 International Council on Clean Transportation This document does not necessarily represent the views of organizations or government agencies represented by ICCT participants. -

Airlines Could Reduce Climate Impact by 10% by Making These Small Changes to Flights 2 March 2017
Airlines could reduce climate impact by 10% by making these small changes to flights 2 March 2017 "With more targeted research, it could become a reality in the next 10 years." Lead author Volker Grewe, from the DLR-Institute of Atmospheric Physics and Professor at the Delft University of Technology, said: "Around 5% of man- made climate change is caused by global aviation, and this number is expected to rise. However, this impact could be reduced if flights were routed to avoid regions where emissions have the largest impact. Credit: University of Reading "Aviation is different from many other sectors, since its climate impact is largely caused by non-CO2 effects, such as contrails and ozone formation. Airlines could reduce their climate impact by up to These non-CO2 effects vary regionally, and, by 10% by making small changes to some flight taking advantage of that, a reduction of aviation's routes, research by University of Reading climate impact is feasible. scientists has shown. "Our study looked at how feasible of such a routing Their new study shows airlines could make a large strategy is. We took into account a representative positive impact on climate change by altering flight set of weather situations for winter and summer, as routes to avoid areas where emissions have the well as safety issues, and optimised all trans- largest impact. The changes would be Atlantic air traffic on those days." comparatively small – resulting in only around a 1% increase in operating costs. Large benefit for small cost An international research team, including Professor Using calculations of emissions, climate change Keith Shine and Dr Emma Irvine from Reading's functions, and air traffic simulations, the research Meteorology Department, alongside experts from team evaluated 85 alternative routes (17 horizontal the DLR Institute of Atmospheric Physics in and five vertical) for each of the roughly 400 flights Germany, Eurocontrol in Brussels, and the Center crossing the North Atlantic in either direction each for International Climate and Environmental day. -

Workshop on the Impacts of Aviation on Climate Change: a Report Of
Joint Planning & Development Office Environmental Integrated Product Team Partnership for AiR Transportation Noise and Emissions Reduction Workshop on the Impacts of Aviation on Climate Change: Add Picture A Report of Findings and Recommendations June 7-9, 2006, Cambridge, MA Executive Summary August 2006 REPORT N0. PARTNER-COE-2006-004-SUMMARY 1 Contents Report Authors................................................................................................................ 2 Acknowledgement........................................................................................................... 3 Executive Summary........................................................................................................ 4 A. Emissions in the UT/LS and Resulting Chemistry Effects ................................. 6 B. Contrails and Cirrus........................................................................................... 7 C. Climate Impacts and Climate Metrics .............................................................. 10 D. Studies for Trade-offs Amongst Aviation Emissions Impacting Climate .......... 12 Appendices....................................................................................................................13 Appendix 1 Workshop Agenda ..............................................................................14 Appendix 2 Workshop Participants/Authors for Each Subgroup............................16 Appendix 3 Other Attendees at the Workshop.......................................................17 -

Accurate Observations of Near-Infrared Solar Spectral Irradiance and Water Vapour Continuum
Accurate observations of near-infrared solar spectral irradiance and water vapour continuum Submitted for the degree of Doctor of Philosophy Department of Meteorology University of Reading Jonathan David Charles Elsey September 2018 Declaration I confirm that this is my own work and the use of all material from other sources has been properly and fully acknowledged. - Jonathan Elsey i Abstract This thesis contains analyses of the solar spectral irradiance (SSI) and near-infrared water vapour continuum from high-resolution observations by a ground-based, sun-pointing Fourier transform spectrometer in the wavenumber region 2000-10000 cm-1 (1-5 µm). This was performed primarily using the Langley method on observations during 18 September 2008. Particular focus was placed on a detailed assessment of the uncertainty budget for each of these analyses. The solar spectral irradiance was found to be ~8% lower than the commonly-used satellite-based ATLAS3 SSI in the region 4000-7000 cm-1 (where ATLAS3 is most uncertain). This disagreement with ATLAS3 is in line with several other modern analyses. There is good agreement with ATLAS3 and other spectra in the 7000-10000 cm-1 region (where these spectra are considered more accurate). This thesis contains the first published results of water vapour continuum absorption in the atmosphere in the 1.6 and 2.1 µm atmospheric windows (in which laboratory measurements show some significant disagreement) with robust uncertainties. The derived water vapour continuum in these windows is stronger than the widely-used MT_CKD model (v3.2) by a factor of ~100 and ~5 respectively. These results also show that MT_CKD is a reasonably accurate representation of the continuum in the 4 µm window.