The Missing Greenhouse Gas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitrogen Trifluoride Hazard Summary Identification
Common Name: NITROGEN TRIFLUORIDE CAS Number: 7783-54-2 RTK Substance number: 1380 DOT Number: UN 2451 Date: March 2001 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY * Nitrogen Trifluoride can affect you when breathed in. * Exposure to hazardous substances should be routinely * Contact may irritate the skin and eyes. evaluated. This may include collecting personal and area * High levels can interfere with the ability of the blood to air samples. You can obtain copies of sampling results carry Oxygen causing headache, fatigue, dizziness, and a from your employer. You have a legal right to this blue color to the skin and lips (methemoglobinemia). information under OSHA 1910.1020. Higher levels can cause trouble breathing, collapse and * If you think you are experiencing any work-related health even death. problems, see a doctor trained to recognize occupational * Repeated high exposure can cause weakness, muscle diseases. Take this Fact Sheet with you. twitching, seizures and convulsions. * Nitrogen Trifluoride may damage the liver and kidneys. WORKPLACE EXPOSURE LIMITS * Repeated high exposure can cause deposits of Fluorides in OSHA: The legal airborne permissible exposure limit the bones and teeth, a condition called "Fluorosis." This (PEL) is 10 ppm averaged over an 8-hour can cause pain, disability and mottling of the teeth. workshift. * The above health effects do NOT occur at the level of Fluoride used in water for preventing cavities in teeth. NIOSH: The recommended airborne exposure limit is 10 ppm averaged over a 10-hour workshift. IDENTIFICATION Nitrogen Trifluoride is a colorless gas with a moldy odor. It ACGIH: The recommended airborne exposure limit is is used as a Fluorine source in the electronics industry and in 10 ppm averaged over an 8-hour workshift. -

NF3, the Greenhouse Gas Missing from Kyoto Michael J
GEOPHYSICAL RESEARCH LETTERS, VOL. 35, L12810, doi:10.1029/2008GL034542, 2008 NF3, the greenhouse gas missing from Kyoto Michael J. Prather1 and Juno Hsu1 Received 5 May 2008; accepted 27 May 2008; published 26 June 2008. [1] Nitrogen trifluoride (NF3) can be called the missing chemical industry are following: Kanto Denka produces greenhouse gas: It is a synthetic chemical produced in 1,000 tons per yr; DuPont is setting up a manufacturing industrial quantities; it is not included in the Kyoto basket of facility in China; Formosa Plastics and Mitsui Chemicals greenhouse gases or in national reporting under the United are expanding production. Data on total production is not Nations Framework Convention on Climate Change freely available, but based on press releases and industry (UNFCCC); and there are no observations documenting its data, we estimate 2008 production to be about 4,000 tons, atmospheric abundance. Current publications report a long with a likely range of ±25%. Planned expansion could lifetime of 740 yr and a global warming potential (GWP), double this by 2010. which in the Kyoto basket is second only to SF6.Were- [3] The published global warming potentials (GWP) of examine the atmospheric chemistry of NF3 and calculate a NF3 are 12,300, 17,200 and 20,700 for time horizons of shorter lifetime of 550 yr, but still far beyond any societal 20, 100, and 500 years, respectively [Forster et al., 2007]. time frames. With 2008 production equivalent to 67 million The increasing GWP with time horizon reflects the longer metric tons of CO2,NF3 has a potential greenhouse impact atmospheric residence time of NF3 compared with that of larger than that of the industrialized nations’ emissions of CO2. -

Nitrogen Trifluoride
Nitrogen trifluoride (CAS No: 7783-54-2) Health-based Reassessment of Administrative Occupational Exposure Limits Committee on Updating of Occupational Exposure Limits, a committee of the Health Council of the Netherlands No. 2000/15OSH/125, The Hague, June 8, 2004 Preferred citation: Health Council of the Netherlands: Committee on Updating of Occupational Exposure Limits. Nitrogen trifluoride; Health-based Reassessment of Administrative Occupational Exposure Limits. The Hague: Health Council of the Netherlands, 2004; 2000/15OSH/125. all rights reserved 1 Introduction The present document contains the assessment of the health hazard of nitrogen trifluoride by the Committee on Updating of Occupational Exposure Limits, a committee of the Health Council of the Netherlands. The first draft of this document was prepared by MA Maclaine Pont, M.Sc. (Wageningen University and Research Centre, Wageningen, the Netherlands). In November 1999, literature was searched in the databases Toxline, Medline, and Chemical Abstracts, starting from 1981, 1966, and 1937, respectively, and using the following key words: nitrogen trifluoride, nitrogen fluoride (NF3), and 7783-54-2. In February 2001, the President of the Health Council released a draft of the document for public review. No comments were received. An additional search in Toxline and Medline in January 2004 did not result in information changing the committee’s conclusions. 2Identity name : nitrogen trifluoride synonyms : nitrogen fluoride; trifluoroamine; trifluoroammonia; perfluoroammonia molecular formula : NF3 CAS number : 7783-54-2 3 Physical and chemical properties molecular weight : 71.0 boiling point : -129oC melting point : -208.5oC flash point : - vapour pressure : at 20°C: >100 kPa solubility in water : very slightly soluble log Poctanol/water : -1.60 conversion factors : at 20°C, 101.3 kPa: 1 mg/m3 = 0.34 ppm 1 ppm = 2.96 mg/m3 Data from ACG91, NLM04, http://esc.syrres.com. -

Cleaning Gas Mixture for an Apparatus and Cleaning Method
Europäisches Patentamt *EP001529854A1* (19) European Patent Office Office européen des brevets (11) EP 1 529 854 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: C23C 16/44, H01L 21/00 11.05.2005 Bulletin 2005/19 (21) Application number: 04256591.1 (22) Date of filing: 26.10.2004 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Isaki, Ryuichiro, HU IE IT LI LU MC NL PL PT RO SE SI SK TR Taiyo Nippon Sanso Corporation Designated Extension States: Shinagawa-ku, Tokyo (JP) AL HR LT LV MK • Shinriki, Manabu, Taiyo Nippon Sanso Corporation (30) Priority: 04.11.2003 JP 2003374160 Shinagawa-ku, Tokyo (JP) (71) Applicant: Taiyo Nippon Sanso Corporation (74) Representative: Smyth, Gyles Darren Shinagawa-ku, Tokyo (JP) Marks & Clerk 90 Long Acre London WC2E 9RA (GB) (54) Cleaning gas mixture for an apparatus and cleaning method (57) A cleaning gas improves the etching reaction and an integer from 1 to 3, y is an integer from 1 to 12, rate of cleaning gas including a fluorocarbon gas, and and z is selected from 0 and 1 and oxygen gas, to which increases the cleaning effect. And the cleaning method is added at least one selected from the group of nitrogen uses the cleaning gas. A mixed gas of a fluorocarbon trifluoride, fluorine, nitrous oxide, nitrogen, and rare gas- gas represented by the general formula of CvHxFyOz, es up to 10% by volume based on the total gas volume. wherein v is an integer from 1 to 5, x is selected from 0 EP 1 529 854 A1 Printed by Jouve, 75001 PARIS (FR) 1 EP 1 529 854 A1 2 Description ciency since these are raw materials for producing fluor- ocarbon type resins such as TEFLON (trademark), and BACKGROUND OF THE INVENTION are used in significantly large amounts in the chemical industry. -

Emissions of Such Gases on a ‘CO2- Equivalent’ Scale
Department of Meteorology Assessing the potential climate impacts of industrial gases Professor Keith Shine FRS | Professor Eleanor Highwood Summary Human activity leads to the emission of many greenhouse gases that differ from carbon dioxide (CO2) in their effects on climate. International climate policy requires the use of an ‘exchange rate’ to place emissions of such gases on a ‘CO2- equivalent’ scale. These ‘exchange rates’ are calculated using ‘climate emission metrics’, which enable quantitative comparisons to be made of the climate impact of the emission of a given gas with respect to CO2 emissions. Background The assessment reports of the Intergovernmental Panel on Climate Change (IPCC) and the World Meteorological Organization / United Nations Environment Programme (WMO/UNEP) Scientific Assessments of Stratospheric Ozone Depletion presented the values of ‘Global Warming Potential’ or GWP. GWP is the metric adopted by the Kyoto Protocol to the United Nations’ Framework Convention on Climate Change (UNFCCC) to allow signatories to report emissions of different greenhouse gases on a CO2-equivalent scale, and is one of a range of possible methods for comparing the climate impact of emissions of different greenhouse gases. How is University of Reading research contributing? Professor Keith Shine played a major role in the international assessments. He led the compilation of databases of an essential input to GWP calculations, namely the so-called ‘radiative efficiency’, or RE, for gases included in the Kyoto Protocol. He and his co-workers within and outside the Department of Meteorology developed and refined methods for calculating RE, using advanced numerical models incorporating new laboratory observations. For many industrial gases, the Reading group has presented the first published RE value, and it has helped resolve instances where results presented in the literature had been in substantive disagreement. -

Metrics for Comparison of Climate Impacts from Well Mixed
1 ACCRI Theme 7 2 3 Metrics for comparison of climate impacts from well mixed greenhouse gases and 4 inhomogeneous forcing such as those from UT/LS ozone, contrails and contrail- 5 cirrus 6 7 Piers Forster & Helen Rogers 8 9 Acknowledgement: The numbers in Table 5 and a many of the ideas are derived from a 10 unpublished manuscript led by Keith Shine that Piers Forster and Helen Rogers were co- 11 author of. 12 13 Executive Summary.......................................................................................................... 2 14 1. Introduction and Background ................................................................................. 5 15 2. Review ........................................................................................................................ 8 16 2.1. Current state of science....................................................................................... 8 17 2.1.1. Air travel – its emissions and its trends ...................................................... 8 18 2.1.2. Aviation’s climate impact......................................................................... 10 19 2.1.3. Review of the RF characteristics and uncertainties of mechanisms ......... 12 20 2.1.3.1. Chemistry of importance to aviation..................................................... 12 21 2.1.3.2. Modelling the impact of aviation.......................................................... 14 22 2.1.4. Regional and timescale issues................................................................... 16 23 2.2. Critical -

Trends and Patterns in the Contributions to Cumulative Radiative Forcing from Different Regions of the World
Trends and patterns in the contributions to cumulative radiative forcing from different regions of the world D. M. Murphya,1 and A. R. Ravishankarab,c,1 aEarth System Research Laboratory, Chemical Sciences Division, National Oceanic and Atmospheric Administration, Boulder, CO 80305; bDepartment of Chemistry, Colorado State University, Fort Collins, CO 80524; and cDepartment of Atmospheric Science, Colorado State University, Fort Collins, CO 80524 Contributed by A. R. Ravishankara, November 12, 2018 (sent for review August 17, 2018; reviewed by John H. Seinfeld and Keith Shine) Different regions of the world have had different historical very short lived, on the order of weeks, so that their radiative patterns of emissions of carbon dioxide, other greenhouse gases, influence is essentially simultaneous with their emission time. and aerosols as well as different land-use changes. One can estimate The relative emissions of GHGs and aerosols have not been the net cumulative contribution by each region to the global mean the same in different regions. One reason is that some regions radiative forcing due to past greenhouse gas emissions, aerosol have had more emissions from industrial activity than other re- precursors, and carbon dioxide from land-use changes. Several gions, and in other regions land-use/land-cover change (LULC) patterns stand out from such calculations. Some regions have had a has been an important driver of emissions. Here we explicitly common historical pattern in which the short-term offsets between deal with only the largest component of the latter––the influence the radiative forcings from carbon dioxide and sulfate aerosols of LULC on CO2. -

1. Hydrogen Forms Compounds with Most Non-Metallic Elements and with Some Metals
PMT 1. Hydrogen forms compounds with most non-metallic elements and with some metals. (a) Calculate the empirical formula of the compound used in the manufacture of artificial rubber which has the following composition by mass. Hydrogen 11.1% Carbon 88.9% (3) (b) The boiling temperatures of hydrogen chloride and hydrogen iodide are: Hydrogen chloride ±85ºC Hydrogen iodide ±35ºC Explain why hydrogen iodide has a higher boiling temperature than hydrogen chloride. ............................................................................................................................. ... ............................................................................................................................. ... ............................................................................................................................. ... (2) (c) Draw and explain the shapes of: (i) the PH3 molecule; .......................................................... ............................................................ ...................................................................................................................... (2) 1 PMT ± (ii) the AlH4 ion. ...................................................................................................................... ...................................................................................................................... (2) 3 (d) Calculate the number of molecules in 8.0 cm of gaseous phosphine, PH3, at room temperature and pressure. (The molar volume of -
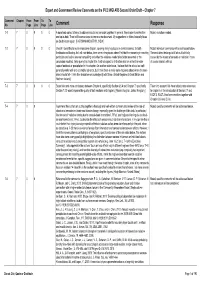
Comment Response 7-1 7 0 0 0 0 a Novel and Correct Attempt to Address Clouds and Aerosols Together
Expert and Government Review Comments on the IPCC WGI AR5 Second Order Draft – Chapter 7 Comment Chapter From From To To No Page Line Page Line Comment Response 7-1 7 0 0 0 0 A novel and correct attempt to address clouds and aerosols together. In general, the chapter is well-written Noted, no action needed. and up-to-date. There still is some scope to improve the document. My suggestions to follow, basically focus on South Asian region [K KRISHNA MOORTHY, INDIA] 7-2 7 0 0 0 0 Overall I found this to be an impressive chapter, covering many key topics in climate science, for both Noted. Individual comments will be addressed below. feedbacks and forcing. As I will note below, there were a few places where I felt that the reasoning in reaching The semi-direct belongs to AFari as it is initially particular conclusions was not compelling and either the evidence needs to be better presented or the caused by the impact of aerosols on radiation. It can conclusion modified. I also query the chapter title. I did not expect to find information on either the water of course interact with aci. vapour feedback or precipitation in this chapter. On another wider issue, I believe that the ari and aci split generally works well and is a helpful advance, but I think there is really some haziness about where the semi- direct should fall - I think this should be acknowledged [Keith Shine, United Kingdom of Great Britain and Northern Ireland] 7-3 7 0 0 0 0 Better links and more consistency between Chapter 6, specifically Section 6.5.4 and Chapter 7, specifically Taken into account. -

Synthetic Greenhouse Gases
Synthetic Greenhouse Gases How are they different to What are ‘synthetic greenhouse gases’? ‘greenhouse gases’? Synthetic greenhouse gases are man made Greenhouse gases are naturally occurring. chemicals. They are commonly used in They include carbon dioxide (CO2), methane refrigeration and air conditioning, fire (CH4) and nitrous oxide (N2O). Synthetic extinguishing, foam production and in greenhouse gases are man made chemicals medical aerosols. When they are released, and generally have a much higher global synthetic greenhouse gases trap heat in warming potential than naturally occurring the atmosphere. greenhouse gases. What is global warming potential? Global warming potential (GWP) is a measure of how much heat a greenhouse gas traps in the atmosphere over a specific time compared to a similar mass of carbon dioxide (CO2). CO2, with a global warming potential of 1, is used as the base figure for measuring global warming potential. The higher the global warming potential number, the more heat a gas traps. The most common synthetic greenhouse gas in Australia is HFC-134a, which is mostly used in refrigerators and air conditioners. It has a global warming potential of 1430; this means the release of one tonne of HFC-134a is equivalent to releasing 1430 tonnes of CO2 into the atmosphere. Which synthetic greenhouse gases does 22,800 Australia regulate? SF6 the global warming potential of sulfur hexafluoride (SF6) • hydrofluorocarbons (HFCs) • perfluorocarbons (PFCs) • sulfur hexafluoride (SF6) Synthetic • nitrogen trifluoride (NF3) greenhouse gases account for around 2% of all greenhouse gas emissions in Australia How do we use synthetic greenhouse gases? They are man made chemicals with a wide variety of uses. -

Heat of Formation of Nitrogen Trifluoride
4j*m *" ARREN i' NATIONAL BUREAU OF STANDARDS REPORT 6363 HEAT OF FORMATION OF NITROGEN TRIFLUORIDE by Sidney Marantz, Charles F* Coyle, and George T. Armstrong Order No. IPR NOrd 03168 Technical Report to U.S. Navy Bureau of Ordnance <NBS> U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions find Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended hy Congress in Public Paw 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to Government Agencies on scientific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. A major portion of the Bureau’s work is performed for other Government Agencies, particularly the Department of Defense and the Atomic Energy Commission. The scope'of activities is suggested by the listing of divisions and sections on the inside of the back cover. Reports and Publications The results of the Bureau’s work take the form of either actual equipment and devices or published papers and reports. Reports are issued to the sponsoring agency of a particular project or program. -

Safety Data Sheet Nitrogen Trifluoride
Safety Data Sheet Nitrogen Trifluoride Section 1: Product and Company Identification Middlesex Gases & Technologies 292 Second Street P.O. Box 490249 Everett, MA 02149 (617) 387-5050 (800) 649-6704 Fax (617) 387-3537 http://www.middlesexgases.com/ Product Code: Nitrogen Trifluoride Section 2: Hazards Identification Warning Hazard Classification: Gases Under Pressure Hazard Statements: Contains gas under pressure; may explode if heated Precautionary Statements Storage: Protect from sunlight. Store in well-ventilated place. Section 3: Composition/Information on Ingredients CAS # 7783-54-2 Middlesex Gases & Technologies page 1 of 4 Generated by the SDS Manager from AsteRisk, LLC. All Rights Reserved Generated: 06/01/2015 Chemical Chemical Trade Names Substance Family Nitrogen Trifluoride inorganic halide Nitrogen fluoride, trifluoroamine, trifluoroammonia, Perfluoroammonia; NF3; UN 2451; N,N,N- Trifluoroamine Section 4: First Aid Measures Skin Contact Eye Contact Ingestion Inhalation Note to Physicians Flush skin with plenty of water for 15 Flush eyes with plenty of Not likely Remove victim to fresh air. Provide Consider minutes. Remove contaminated clothing water for 15 minutes. Get route of artificial respiration if breathing is oxygen. and shoes, wash before reuse. Get medical attention exposure. difficult. Consider oxygen. Contact medical attention immediately. immediately. medical personnel immediately. Section 5: Fire Fighting Measures Suitable Extinguishing Media Products of Protection of Firefighters Combustion Non-flammable. Use extinguishing media suitable for Non-flammable § Wear self-contained breathing apparatus. surrounding fire. Section 6: Accidental Release Measures Personal Precautions Environmental Precautions Methods for Containment Isolate area. Contact emergency personnel. Eliminate ignition Avoid contact with soil, waterways, drains Shut off flow if possible sources if it is safe to do so.