W9646-Front Matter W9646-Front Matter.Qxd
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
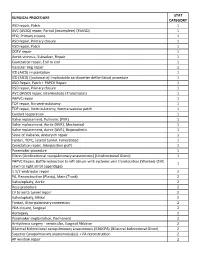
Surgeries by STAT Category
STAT SURGICAL PROCEDURE CATEGORY ASD repair, Patch 1 AVC (AVSD) repair, Partial (Incomplete) (PAVSD) 1 PFO, Primary closure 1 ASD repair, Primary closure 1 VSD repair, Patch 1 DCRV repair 1 Aortic stenosis, Subvalvar, Repair 1 Coarctation repair, End to end 1 Vascular ring repair 1 ICD (AICD) implantation 1 ICD (AICD) ([automatic] implantable cardioverter deFibrillator) procedure 1 ASD Repair, Patch + PAPCV Repair 1 VSD repair, Primary closure 1 AVC (AVSD) repair, Intermediate (Transitional) 1 PAPVC repair 1 TOF repair, No ventriculotomy 1 TOF repair, Ventriculotomy, Nontransanular patch 1 Conduit reoperation 1 Valve replacement, Pulmonic (PVR) 1 Valve replacement, Aortic (AVR), Mechanical 1 Valve replacement, Aortic (AVR), Bioprosthetic 1 Sinus oF Valsalva, Aneurysm repair 1 Fontan, TCPC, Lateral tunnel, Fenestrated 1 Coarctation repair, Interposition graFt 1 Pacemaker procedure 1 Glenn (Unidirectional cavopulmonary anastomosis) (Unidirectional Glenn) 1 PAPVC Repair, BaFFle redirection to leFt atrium with systemic vein translocation (Warden) (SVC 1 sewn to right atrial appendage) 1 1/2 ventricular repair 2 PA, Reconstruction (Plasty), Main (Trunk) 2 Valvuloplasty, Aortic 2 Ross procedure 2 LV to aorta tunnel repair 2 Valvuloplasty, Mitral 2 Fontan, Atrio-pulmonary connection 2 PDA closure, Surgical 2 Aortopexy 2 Pacemaker implantation, Permanent 2 Arrhythmia surgery - ventricular, Surgical Ablation 2 Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (Bilateral bidirectional Glenn) 2 Superior Cavopulmonary anastomosis(es) + PA -

Heart Disease in Children.Pdf
Heart Disease in Children Richard U. Garcia, MD*, Stacie B. Peddy, MD KEYWORDS Congenital heart disease Children Primary care Cyanosis Chest pain Heart murmur Infective endocarditis KEY POINTS Fetal and neonatal diagnosis of congenital heart disease (CHD) has improved the out- comes for children born with critical CHD. Treatment and management of CHD has improved significantly over the past 2 decades, allowing more children with CHD to grow into adulthood. Appropriate diagnosis and treatment of group A pharyngitis and Kawasaki disease in pe- diatric patients mitigate late complications. Chest pain, syncope, and irregular heart rhythm are common presentations in primary care. Although typically benign, red flag symptoms/signs should prompt a referral to car- diology for further evaluation. INTRODUCTION The modern incidence of congenital heart disease (CHD) has been reported at 6 to 11.1 per 1000 live births.1,2 The true incidence is likely higher because many miscar- riages are due to heart conditions incompatible with life. The unique physiology of CHD, the constantly developing nature of children, the differing presenting signs and symptoms, the multiple palliative or corrective surgeries, and the constant devel- opment of new strategies directed toward improving care in this population make pe- diatric cardiology an exciting field in modern medicine. THE FETAL CIRCULATION AND TRANSITION TO NEONATAL LIFE Cardiovascular morphogenesis is a complex process that transforms an initial single- tube heart to a 4-chamber heart with 2 separate outflow tracts. Multiple and Disclosure Statement: All Authors take responsibility for all aspects of the reliability and freedom from bias of the information presented and their discussed interpretation. -

Interstage Monitoring for the Infant with Hypoplastic Left Heart Syndrome What the Direct Care Nurse Needs to Know Jennifer Stra
Interstage Monitoring for the Infant with Hypoplastic Left Heart Syndrome What the Direct Care Nurse Needs to Know Jennifer Strawn, BSN, RN, CPN, Children’s Hospital & Medical Center, Omaha, Nebraska Jo Ann Nieves MSN, CPN, ARNP, PNP-BC, FAHA, Miami Children’s Hospital Bronwyn Bartle, DNP, CPNP-AC/PC, Duke Children’s Pediatric and Congenital Heart Center Mary Rummell, MN, RN, CNS, CPNP, FAHA, Oregon Health and Science University Introduction: Children born with hypoplastic left heart syndrome (HLHS) are at high risk for serious morbidity, growth failure and mortality during the time from discharge home after first stage HLHS palliation, until admission for the second stage surgical intervention. To improve outcomes and survival during this time, referred to as the “interstage period”, multiple strategies have been successfully implemented resulting in improved interstage survival. A critical element of interstage care is family education and training. Variations in practice and interstage home surveillance do exist but general guidelines are described below. Definitions: Possible newborn interventions for HLHS Figure 1: First stage surgical palliation procedures for hyoplastic left heart syndrome A. Norwood Procedure B. Norwood Procedure with C. Hybrid Stage I with BT shunt RV to PA shunt (“Sano”) Procedure Surgical: Norwood /Modified Blalock Taussig (BT) shunt procedure – the Norwood aortic reconstruction of the ascending aorta and arch consists of enlarging the ascending aorta by using part of the proximal native main pulmonary artery (PA) with other material (Gore-Tex or homograft material) to connect the PA to the aorta. Pulmonary blood flow is provided by a controlled shunt (BT shunt) usually made from Gore-Tex and connects the right subclavian artery to the right pulmonary artery. -

Long Term Consequences of the Fontan Procedure and How to Manage Them
ACCEPTED MANUSCRIPT Long Term Consequences of the Fontan Procedure and How to Manage Them Authors: W. Aaron Kay, MD1 Tabitha Moe, MD2 Blair Suter, MD3 Andrea Tennancour, NP1 Alice Chan, NP4 Richard A. Krasuski, MD5 Ali N. Zaidi, MD4 1. Indiana University School of Medicine, Krannert Institute of Cardiology, IN 2. University of Arizona School of Medicine, Phoenix, AZ 3. Indiana University School of Medicine, Departments of Medicine and Pediatrics, IN 4. Children’s Hospital at Montefiore, Montefiore Medical Center, Albert Einstein College of Medicine, NY 5. Duke University Health System, Durham, NC *Address reprint requests to: W. Aaron Kay, MD. 1801 N. Senate Blvd Suite 2000, Indianapolis IN. Email: [email protected] Emails of additional authors: Moe TG: [email protected] Suter BC: [email protected] Tennancour AE: [email protected] Chan A: [email protected] Krasuski RA: [email protected] Zaidi AN: [email protected] Disclosures: W. Aaron Kay: Nothing to disclose Tabitha Moe: Nothing to disclose Blair Suter, MD: Nothing to disclose Andrea Tennancour, NP: Nothing to disclose Alice Chan, NP: Nothing to disclose Ali N. Zaidi, MD: Nothing to disclose Richard A. Krasuski, MD: Dr. Krasuski serves a consultant and receives research funding from Actelion Pharmaceuticals.ACCEPTED He also serves as anMANUSCRIPT investigator for Edwards Lifesciences and is an unpaid member of the scientific advisory board for Ventripoint. ___________________________________________________________________ This is the author's manuscript of the article published in final edited form as: Kay, W. A., Moe, T., Suter, B., Tennancour, A., Chan, A., Krasuski, R. A., & Zaidi, A. N. (2018). Long Term Consequences of the Fontan Procedure and How to Manage Them. -

Comparison of Hybrid and Norwood Strategies in Hypoplastic Left Heart Syndrome
SURGERY | REVIEW Comparison of Hybrid and Norwood Strategies in Hypoplastic Left Heart Syndrome Hideyuki Kato, MD, Osami Honjo, MD, PhD, Glen Van Arsdell, MD, Christopher A. Caldarone, MD Division of Cardiovascular Surgery, Hospital for Sick Children, Labatt Family Heart Center Received 12/12/2011, Reviewed 27/12/2011, Accepted 1/1/2012 Key words: Hybrid strategy, Norwood strategy, single ventricle, hypoplastic left heart syndrome DOI: 10.5083/ejcm.20424884.70 ABSTRACT CORRESPONDENCE Current surgical palliation for neonates with single ventricle physiology includes Norwood-based Christopher A. Caldarone, MD, and Hybrid-based surgical strategies. When transplantation is not available, clinicians must choose Professor and Chair, Division of Cardiac Surgery, between these two strategies with distinctly different learning curves and risk profiles. The Norwood University of Toronto, strategy has evolved over several decades while the rising popularity of the Hybrid strategy is a Watson Family Chair in much more recent addition to our therapeutic options. Based upon the premise that avoiding cardio Cardiovascular Science – pulmonary bypass in the neonatal period and deferral of aortic arch reconstruction until a second Labatt Family Heart Center, stage procedure have an important influence on outcomes, the Hybrid strategy has compelling Hospital for Sick Children, theoretical advantages and disadvantages in comparison to the Norwood strategy. The purpose 555 University Avenue, of this review is to summarise the currently available data to support -

Risk Factors for Mortality After the Norwood Procedureq
European Journal of Cardio-thoracic Surgery 22 (2002) 82–89 www.elsevier.com/locate/ejcts Risk factors for mortality after the Norwood procedureq J. William Gaynora,*, William T. Mahleb, Mitchell I. Cohenb, Richard F. Ittenbachc, William M. DeCamplia, James M. Stevend, Susan C. Nicolsond, Thomas L. Spraya Downloaded from https://academic.oup.com/ejcts/article/22/1/82/516394 by guest on 28 September 2021 aDivision of Cardiothoracic Surgery, The Cardiac Center at The Children’s Hospital of Philadelphia, 34th Street and Civic Center Boulevard, Suite 8527, Philadelphia, PA 19104, USA bDivision of Cardiology, The Cardiac Center at The Children’s Hospital of Philadelphia, Philadelphia, PA, USA cDivision of Biostatistics and Epidemiology, The Cardiac Center at The Children’s Hospital of Philadelphia, Philadelphia, PA, USA dDivision of Cardiac Anesthesiology, The Cardiac Center at The Children’s Hospital of Philadelphia, Philadelphia, PA, USA Received 18 September 2001; received in revised form 7 March 2002; accepted 22 March 2002 Abstract Objectives: Recent studies have suggested that survival following the Norwood procedure is influenced by anatomy and is worse for patients with hypoplastic left heart syndrome (HLHS), particularly aortic atresia (AA), as compared to other forms of functional single ventricle and systemic outflow tract obstruction. The current study was undertaken to evaluate our recent experience with the Norwood procedure and to evaluate potential predictors of operative and 1-year mortality. Methods: A retrospective study of risk factors for operative and 1-year mortality in 158 patients undergoing the Norwood procedure between January 1, 1998 and June 30, 2001. Results: HLHS was present in 102 patients (70 with AA) and other forms of functional single ventricle with systemic outflow tract obstruction in the remaining 56. -

Chest Pain in Children and Adolescents Surendranath R
Article cardiology Chest Pain in Children and Adolescents Surendranath R. Veeram Objectives After completing this article, readers should be able to: Reddy, MD,* Harinder R. Singh, MD* 1. Enumerate the most common causes of chest pain in pediatric patients. 2. Differentiate cardiac chest pain from that of noncardiac cause. 3. Describe the detailed evaluation of a pediatric patient who has chest pain. Author Disclosure 4. Screen and identify patients who require a referral to a pediatric cardiologist or other Drs Veeram Reddy specialist. and Singh have 5. Explain the management of the common causes of pediatric chest pain. disclosed no financial relationships relevant Case Studies to this article. This Case 1 commentary does not During an annual physical examination, a 12-year-old girl complains of intermittent chest contain a discussion pain for the past 5 days that localizes to the left upper sternal border. The pain is sharp and of an unapproved/ stabbing, is 5/10 in intensity, increases with deep breathing, and lasts for less than 1 minute. investigative use of a The patient has no history of fever, cough, exercise intolerance, palpitations, dizziness, or commercial product/ syncope. On physical examination, the young girl is in no pain or distress and has normal vital signs for her age. Examination of her chest reveals no signs of inflammation over the sternum device. or rib cage. Palpation elicits mild-to-moderate tenderness over the left second and third costochondral junctions. The patient reports that the pain during the physical examination is similar to the chest pain she has experienced for the past 5 days. -

The Intensive Care of Infants with Hypoplastic Left Heart Syndrome
F97 Arch Dis Child Fetal Neonatal Ed: first published as 10.1136/adc.2004.064337 on 21 February 2005. Downloaded from RECENT ADVANCES The intensive care of infants with hypoplastic left heart syndrome U Theilen, L Shekerdemian ............................................................................................................................... Arch Dis Child Fetal Neonatal Ed 2005;90:F97–F102. doi: 10.1136/adc.2004.051276 Until a little over two decades ago, hypoplastic left heart excessive pulmonary blood flow, an important subgroup may have inadequate inter-atrial mix- syndrome was considered an inoperable and fatal ing due to a restrictive atrial septal defect (ASD). condition, with most deaths occurring in early infancy, and This results in global hypoperfusion, with pro- almost all of those affected dying before their first birthday. found hypoxaemia and acidosis, severe pulmon- ary venous hypertension, and overwhelming However, the advent of surgical palliation and advances in pulmonary venous congestion on chest x ray. peri-operative care, have offered hope to these patients These infants are generally unresponsive to and their families. measures aimed at improving pulmonary flow such as aggressive mechanical ventilation with ........................................................................... high inspired oxygen fractions or nitric oxide, and if left untreated they rapidly develop ypoplastic left heart syndrome (HLHS) is a progressive pulmonary venous hypertension. continuum which can affect all left sided This is associated with increased mortality in Hcardiac structures, from the mitral valve to infants with HLHS.34 the aortic arch. Since Norwood’s first description A restrictive ASD requires urgent intervention. of surgical palliation in 1981,1 HLHS has been If this has been diagnosed antenatally, delivery managed either by staged palliation in the should be planned in a centre able to urgently majority of cases, or, in a minority, by primary institute cardiopulmonary bypass. -

Norwood/Batista Operation for a Newborn with Dilated Myopathy of the Left Ventricle
612 Brief communications The Journal of Thoracic and Cardiovascular Surgery September 2000 NORWOOD/BATISTA OPERATION FOR A NEWBORN WITH DILATED MYOPATHY OF THE LEFT VENTRICLE Richard D. Mainwaring, MD, Regina M. Healy, BS, John D. Murphy, MD, and William I. Norwood, MD, PhD, Wilmington, Del Partial left ventriculectomy for dilated cardiomyopathy was contractile function. The variable results that have been first reported by Batista and associates1 in 1996. The rationale reported in the adult literature may reflect patient selection for this procedure is the increase in left ventricular cavity size according to the reversibility or recoverability of the underly- in the absence of compensatory left ventricular wall thickness ing disease process. that is observed in dilated cardiomyopathy. This combination Despite the substantial worldwide experience with the of factors results in an increase in wall stress per unit muscle Batista procedure in adult patients, there is limited experience mass, as predicted by the LaPlace equation. As wall stress with this procedure in children. The current case report increases, mechanical load eventually becomes nonsustain- describes the treatment of a patient in whom the diagnosis of able and contributes to further dilation of the ventricle. Partial dilated cardiomyopathy was made in utero. left ventriculectomy has been advocated as a method of Clinical summary. A female infant was recognized in restoring the balance between cavity size and wall thickness. utero as having a dilated, poorly functioning left ventricle. Fundamental to this concept is the assumption that the left Labor was induced at 36 weeks’ gestation, and she was deliv- ventricular muscle is intrinsically normal or has recoverable ered by normal, spontaneous, vaginal delivery. -

Chest Pain in Pediatrics
PEDIATRIC CARDIOLOGY 0031-3955/99 $8.00 + .OO CHEST PAIN IN PEDIATRICS Keith C. Kocis, MD, MS Chest pain is an alarming complaint in children, leading an often frightened and concerned family to a pediatrician or emergency room and commonly to a subsequent referral to a pediatric cardiologist. Because of the well-known associ- ation of chest pain with significant cardiovascular disease and sudden death in adult patients, medical personnel commonly share heightened concerns over pediatric patients presenting with chest pain. Although the differential diagnosis of chest pain is exhaustive, chest pain in children is least likely to be cardiac in origin. Organ systems responsible for causing chest pain in children include*: Idiopathic (12%-85%) Musculoskeletal (15%-31%) Pulmonary (12%-21%) Other (4%-21%) Psychiatric (5%-17%) Gastrointestinal (4'/0-7%) Cardiac (4%4%) Furthermore, chest pain in the pediatric population is rareZy associated with life-threatening disease; however, when present, prompt recognition, diagnostic evaluation, and intervention are necessary to prevent an adverse outcome. This article presents a comprehensive list of differential diagnostic possibilities of chest pain in pediatric patients, discusses the common causes in further detail, and outlines a rational diagnostic evaluation and treatment plan. Chest pain, a common complaint of pediatric patients, is often idiopathic in etiology and commonly chronic in nature. In one study,67 chest pain accounted for 6 in 1000 visits to an urban pediatric emergency room. In addition, chest pain is the second most common reason for referral to pediatric cardiologist^.^, 23, 78 Chest pain is found equally in male and female patients, with an average *References 13, 17, 23, 27, 32, 35, 44, 48, 49, 63-67, 74, and 78. -

Identifying and Treating Chest Pain
Identifying and Treating Chest Pain The Congenital Heart Collaborative Cardiac Chest Pain University Hospitals Rainbow Babies & Children’s Hospital Chest pain due to a cardiac condition is rare in children and and Nationwide Children’s Hospital have formed an innovative adolescents, with a prevalence of less than 5 percent. The affiliation for the care of patients with congenital heart disease cardiac causes of chest pain include inflammation, coronary from fetal life to adulthood. The Congenital Heart Collaborative insufficiency, tachyarrhythmias, left ventricular outflow tract provides families with access to one of the most extensive and obstruction and connective tissue abnormalities. experienced heart teams – highly skilled in the delivery of quality clinical services, novel therapies and a seamless continuum of care. Noncardiac Chest Pain Noncardiac chest pain is, by far, the most common cause of chest pain in children and adolescents, accounting for 95 percent of Pediatric Chest Pain concerns. Patients are often unnecessarily referred to a pediatric In pediatrics, chest pain has a variety of symptomatic levels and cardiologist for symptoms. This causes increased anxiety and causes. It can range from a sharp stab to a dull ache; a crushing distress within the family. Noncardiac causes of chest pain are or burning sensation; or even pain that travels up to the neck, musculoskeletal, pulmonary, gastrointestinal and miscellaneous. jaw and back. Chest pain can be cause for alarm in both patients The most common cause of chest pain in children and and parents, and it warrants careful examination and treatment. adolescents is musculoskeletal or chest-wall pain. Pediatric chest pain can be broadly classified as cardiac chest pain Reassurance, rest and analgesia are the primary treatments or noncardiac chest pain. -

Kounis Syndrome: a Forgotten Cause of References Chest Pain/ Cardiac Chest Pain in Children 1
382 Editöre Mektuplar Letters to the Editor Kounis syndrome: A forgotten cause of References chest pain/ Cardiac chest pain in children 1. Çağdaş DN, Paç FA. Cardiac chest pain in children. Anadolu Kardiyol Derg 2009;9:401-6. Kounis sendromu: Göğüs ağrısının unutulan bir sebebi/ 2. Coleman WL. Recurrent chest pain in children. Pediatr Clin North Am Çocuklarda kardiyak göğüs ağrısı 1984;31:1007-26. 3. Kounis NG. Kounis syndrome (allergic angina and allergic myocardial infarction): a natural paradigm? Int J Cardiol 2006; 7:7-14. Dear Editor, 4. Biteker M, Duran NE, Biteker F, Civan HA, Gündüz S, Gökdeniz T, et al. Kounis syndrome: first series in Turkish patients. Anadolu Kardiyol Derg I read with interest the article “Cardiac chest pain in children” by 2009;9:59-60. Çağdaş et al. (1) which has retrospectively evaluated 120 children 5. Biteker M. A new classification of Kounis syndrome. Int J Cardiol 2010 Jun admitted to a pediatric cardiology clinic with chest pain. Although chest 7. [Epub ahead of print]. pain in children is rarely reported to be associated with cardiac 6. Biteker M, Duran NE, Ertürk E, Aykan AC, Civan HA, et al. Kounis Syndrome diseases in the literature (2) authors have found that 52 of the patients secondary to amoxicillin/clavulanic acid use in a child. Int J Cardiol 2009;136:e3-5. (42.5%) had cardiac diseases and 28 (23.3%) of these patients’ cardiac 7. Biteker M, Ekşi Duran N, Sungur Biteker F, Ayyıldız Civan H, Kaya H, diseases were thought to directly cause their chest pain.