Nature Template
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
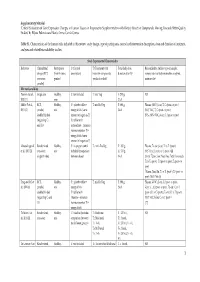
Critical Evaluation of Gene Expression Changes in Human Tissues In
Supplementary Material ‘Critical Evaluation of Gene Expression Changes in Human Tissues in Response to Supplementation with Dietary Bioactive Compounds: Moving Towards Better-Quality Studies’ by Biljana Pokimica and María-Teresa García-Conesa Table S1. Characteristics of the human trials included in this review: study design, type of participants, control and intervention description, dose and duration of treatment, analyses and related bioavailability studies. Study Experimental Characteristics Reference Clinical trial Participants C (Control T (Treatment with Total daily dose, Bioavailability studies: type of sample, design (RCT, (health status, description) bioactive compounds, duration (d or h)1 compounds and (or) metabolites analysed, crossover, gender) products or diet) main results2 parallel) Mix meals and diets Persson I et al., Single arm Healthy, C: not included T: mix Veg T: 250 g, NR 2000 [1] men 21 d Møller P et al., RCT, Healthy, C1: placebo tablet + T: mix FruVeg T: 600 g, Plasma: (NS↑) β-car, T, C2 (post- vs pre-) 2003 [2] parallel, mix energy drink (same 24 d (NC) VitC, T, C2 (post- vs pre-) double blinded amount of sugars as T) (NS↓, 69%) VitC, β-car, C1 (post- vs pre-) (regarding C1 C2: tablet with and C2) antioxidants + minerals (same amount as T) + energy drink (same amount of sugars as T) Almendingen K Randomized, Healthy, C: no proper control T1,2: mix FruVeg T1: 300 g, Plasma: ↑α-car, β-car, T2 vs T1 (post-) et al., 2005 [3] crossover, mix included (comparison T2: 750 g, (NS↑) Lyc, Lut, T2 vs T1 (post-) [4] single -

Gene Expression Studies: from Case-Control to Multiple-Population-Based Studies
From the Institute of Human Genetics, Helmholtz Zentrum Munchen,¨ Deutsches Forschungszentrum fur¨ Gesundheit und Umwelt (GmbH) Head: Prof. Dr. Thomas Meitinger Gene expression studies: From case-control to multiple-population-based studies Thesis Submitted for a Doctoral Degree in Natural Sciences at the Faculty of Medicine, Ludwig-Maximilians-Universitat¨ Munchen¨ Katharina Schramm Dachau, Germany 2016 With approval of the Faculty of Medicine Ludwig-Maximilians-Universit¨atM ¨unchen Supervisor/Examiner: Prof. Dr. Thomas Illig Co-Examiners: Prof. Dr. Roland Kappler Dean: Prof. Dr. med. dent. Reinhard Hickel Date of oral examination: 22.12.2016 II Dedicated to my family. III Abstract Recent technological developments allow genome-wide scans of gene expression levels. The reduction of costs and increasing parallelization of processing enable the quantification of 47,000 transcripts in up to twelve samples on a single microarray. Thereby the data collec- tion of large population-based studies was improved. During my PhD, I first developed a workflow for the statistical analyses of case-control stu- dies of up to 50 samples. With large population-based data sets generated I established a pipeline for quality control, data preprocessing and correction for confounders, which re- sulted in substantially improved data. In total, I processed more than 3,000 genome-wide expression profiles using the generated pipeline. With 993 whole blood samples from the population-based KORA (Cooperative Health Research in the Region of Augsburg) study we established one of the largest population-based resource. Using this data set we contributed to a number of transcriptome-wide association studies within national (MetaXpress) and international (CHARGE) consortia. -

Exploring Prostate Cancer Genome Reveals Simultaneous Losses of PTEN, FAS and PAPSS2 in Patients with PSA Recurrence After Radical Prostatectomy
Int. J. Mol. Sci. 2015, 16, 3856-3869; doi:10.3390/ijms16023856 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Exploring Prostate Cancer Genome Reveals Simultaneous Losses of PTEN, FAS and PAPSS2 in Patients with PSA Recurrence after Radical Prostatectomy Chinyere Ibeawuchi 1, Hartmut Schmidt 2, Reinhard Voss 3, Ulf Titze 4, Mahmoud Abbas 5, Joerg Neumann 6, Elke Eltze 7, Agnes Marije Hoogland 8, Guido Jenster 9, Burkhard Brandt 10 and Axel Semjonow 1,* 1 Prostate Center, Department of Urology, University Hospital Muenster, Albert-Schweitzer-Campus 1, Gebaeude 1A, Muenster D-48149, Germany; E-Mail: [email protected] 2 Center for Laboratory Medicine, University Hospital Muenster, Albert-Schweitzer-Campus 1, Gebaeude 1A, Muenster D-48149, Germany; E-Mail: [email protected] 3 Interdisciplinary Center for Clinical Research, University of Muenster, Albert-Schweitzer-Campus 1, Gebaeude D3, Domagkstrasse 3, Muenster D-48149, Germany; E-Mail: [email protected] 4 Pathology, Lippe Hospital Detmold, Röntgenstrasse 18, Detmold D-32756, Germany; E-Mail: [email protected] 5 Institute of Pathology, Mathias-Spital-Rheine, Frankenburg Street 31, Rheine D-48431, Germany; E-Mail: [email protected] 6 Institute of Pathology, Klinikum Osnabrueck, Am Finkenhuegel 1, Osnabrueck D-49076, Germany; E-Mail: [email protected] 7 Institute of Pathology, Saarbrücken-Rastpfuhl, Rheinstrasse 2, Saarbrücken D-66113, Germany; E-Mail: [email protected] 8 Department -

SMARCA1 Antibody A
Revision 1 C 0 2 - t SMARCA1 Antibody a e r o t S Orders: 877-616-CELL (2355) [email protected] Support: 877-678-TECH (8324) 0 5 Web: [email protected] 4 www.cellsignal.com 9 # 3 Trask Lane Danvers Massachusetts 01923 USA For Research Use Only. Not For Use In Diagnostic Procedures. Applications: Reactivity: Sensitivity: MW (kDa): Source: UniProt ID: Entrez-Gene Id: WB, IP H Endogenous 130 Rabbit P28370 6594 Product Usage Information 5. Ho, L. and Crabtree, G.R. (2010) Nature 463, 474-84. 6. Landry, J.W. et al. (2011) Genes Dev 25, 275-86. Application Dilution 7. Landry, J. et al. (2008) PLoS Genet 4, e1000241. Western Blotting 1:1000 Immunoprecipitation 1:50 Storage Supplied in 10 mM sodium HEPES (pH 7.5), 150 mM NaCl, 100 µg/ml BSA and 50% glycerol. Store at –20°C. Do not aliquot the antibody. Specificity / Sensitivity SMARCA1 Antibody recognizes endogenous levels of total SMARCA1 protein. Species Reactivity: Human Source / Purification Polyclonal antibodies are produced by immunizing animals with a synthetic peptide corresponding to residues near the amino terminus of human SMARCA1 protein. Antibodies are purified by protein A and peptide affinity chromatography. Background SMARCA1 (SNF2L) is one of the two orthologs of the ISWI (imitation switch) ATPases encoded by the mammalian genome (1). The ISWI chromatin remodeling complexes were first identified in Drosophila and have been shown to remodel and alter nucleosome spacing in vitro (2). SMARCA1 is the catalytic subunit of the nucleosome remodeling factor (NURF) and CECR2-containing remodeling factor (CERF) complexes (3-5). -

Monoclonal Antibody to SMARCA1
AM50455PU-N OriGene Technologies Inc. OriGene EU Acris Antibodies GmbH 9620 Medical Center Drive, Ste 200 Schillerstr. 5 Rockville, MD 20850 32052 Herford UNITED STATES GERMANY Phone: +1-888-267-4436 Phone: +49-5221-34606-0 Fax: +1-301-340-8606 Fax: +49-5221-34606-11 [email protected] [email protected] Monoclonal Antibody to SMARCA1 - Purified Alternate names: ATP-dependent helicase SMARCA1, Nucleosome-remodeling factor subunit SNF2L, Probable global transcription activator SNF2L1, SNF2L, SNF2L1, SWI/SNF-related matrix- associated actin-dependent regulator of chromatin subfamily A member 1 Catalog No.: AM50455PU-N Quantity: 0.1 mg Concentration: lot-specific Background: Nucleosome-remodeling factor subunit SNF2L, also known as SWI/SNF-related matrix- associated actin-dependent regulator of chromatin subfamily A member 1 (SMARCA1), is the energy-transducing component of NURF (nucleosome-remodeling factor) and CERF (CECR2-containing-remodeling factor) complexes. These complexes facilitate the disruption of chromatin structure in an ATP-dependent manner. SNF2L potentiates neurite outgrowth, and may be involved in brain development by regulating En-1 and En-2 expression as well as in the development of luteal cells. Uniprot ID: P28370 NCBI: NP_003060.2 GeneID: 6594 Host / Isotype: Rat / IgG2b Clone: SNF 2C4 Immunogen: GST-tagged recombinant protein corresponding to human SNF2L. Format: State: Liquid purified Ig fraction Purification: Protein G Chromatography Buffer System: 0.1 M Tris-Glycine (pH 7.4), 150 mM NaCl with 0.05% sodium azide. Applications: Immunohistochemistry: A representative lot was used by an an independent laboratory to detect SNF2L in certain, normal human organ tissues. (Eckey, M., et al. -

SMARCA1 (D4Q7V) Rabbit
SMARCA1 (D4Q7V) Rabbit mAb Store at -20°C 3 n 100 µl Orders n 877-616-CELL (2355) (10 western blots) [email protected] Support n 877-678-TECH (8324) [email protected] Web n www.cellsignal.com New 03/13 #12483 For Research Use Only. Not For Use In Diagnostic Procedures. Entrez-Gene ID #6594 Swiss-Prot Acc. #P28370 Storage: Supplied in 10 mM sodium HEPES (pH 7.5), 150 Applications Species Cross-Reactivity* Molecular Wt. Isotype mM NaCl, 100 µg/ml BSA, 50% glycerol and less than 0.02% W, IP H, Mk 130 kDa Rabbit IgG** sodium azide. Store at –20°C. Do not aliquot the antibody. Endogenous *Species cross-reactivity is determined by western blot. Background: SMARCA1 (SNF2L) is one of the two ortho- ** Anti-rabbit secondary antibodies must be used to logs of the ISWI (imitation switch) ATPases encoded by the detect this antibody. kDa LN18 SW620 HeLa HT-29 Saos-2 OVCAR8COS-7 mammalian genome (1). The ISWI chromatin remodeling Recommended Antibody Dilutions: complexes were first identified in Drosophila and have been 200 140 Western blotting 1:1000 shown to remodel and alter nucleosome spacing in vitro (2). SMARCA1 100 Immunoprecipitation 1:100 SMARCA1 is the catalytic subunit of the nucleosome re- 80 modeling factor (NURF) and CECR2-containing remodeling For product specific protocols please see the web page 60 factor (CERF) complexes (3-5). The NURF complex plays an 50 for this product at www.cellsignal.com. important role in neuronal physiology by promoting neurite 40 Please visit www.cellsignal.com for a complete listing outgrowth and regulation of Engrailed homeotic genes that 30 of recommended complementary products. -

Contribution of Multiple Inherited Variants to Autism Spectrum Disorder (ASD) in a Family with 3 Affected Siblings
G C A T T A C G G C A T genes Article Contribution of Multiple Inherited Variants to Autism Spectrum Disorder (ASD) in a Family with 3 Affected Siblings Jasleen Dhaliwal 1 , Ying Qiao 1,2, Kristina Calli 1,2, Sally Martell 1,2, Simone Race 3,4,5, Chieko Chijiwa 1,5, Armansa Glodjo 3,5, Steven Jones 1,6 , Evica Rajcan-Separovic 2,7, Stephen W. Scherer 4 and Suzanne Lewis 1,2,5,* 1 Department of Medical Genetics, University of British Columbia (UBC), Vancouver, BC V6H 3N1, Canada; [email protected] (J.D.); [email protected] (Y.Q.); [email protected] (K.C.); [email protected] (S.M.); [email protected] (C.C.); [email protected] (S.J.) 2 BC Children’s Hospital, Vancouver, BC V5Z 4H4, Canada; [email protected] 3 Department of Pediatrics, University of British Columbia (UBC), Vancouver, BC V6T 1Z7, Canada; [email protected] (S.R.); [email protected] (A.G.) 4 The Centre for Applied Genomics and McLaughlin Centre, Hospital for Sick Children and University of Toronto, Toronto, ON M5G 0A4, Canada; [email protected] 5 BC Children’s and Women’s Health Center, Vancouver, BC V6H 3N1, Canada 6 Michael Smith Genome Sciences Centre, Vancouver, BC V5Z 4S6, Canada 7 Department of Pathology and Laboratory Medicine, University of British Columbia (UBC), Vancouver, BC V6T 1Z7, Canada * Correspondence: [email protected] Abstract: Autism Spectrum Disorder (ASD) is the most common neurodevelopmental disorder in Citation: Dhaliwal, J.; Qiao, Y.; Calli, children and shows high heritability. -

Dematin (18): Sc-135881
SAN TA C RUZ BI OTEC HNOL OG Y, INC . Dematin (18): sc-135881 BACKGROUND APPLICATIONS Caldesmon, Filamin 1, Nebulin, Villin, Plastin, ADF, Gelsolin, Dematin and Dematin (18) is recommended for detection of Dematin of mouse, rat and Cofilin are differentially expressed Actin binding proteins. Dematin is a human origin by Western Blotting (starting dilution 1:200, dilution range bundling protein of the erythrocyte membrane skeleton. Dematin is localized 1:100-1:1000), immunoprecipitation [1-2 µg per 100-500 µg of total protein to the spectrin-Actin junctions and its Actin-bundling activity is abolished (1 ml of cell lysate)] and immunofluorescence (starting dilution 1:50, dilution upon phosphorylation by cAMP-dependent protein kinase. It may also play a range 1:50-1:500). role in the regulation of cell shape, implying a role in tumorigenesis. Dematin Suitable for use as control antibody for Dematin siRNA (h): sc-105286, is a trimeric protein containing two identical subunits and a larger subunit. Dematin siRNA (m): sc-142992, Dematin shRNA Plasmid (h): sc-105286-SH, It is localized to the heart, brain, lung, skeletal muscle and kidney. The Dematin shRNA Plasmid (m): sc-142992-SH, Dematin shRNA (h) Lentiviral Dematin gene is located on human chromosome 8p21, a region frequently Particles: sc-105286-V and Dematin shRNA (m) Lentiviral Particles: delet ed in prostate cancer, and mouse chromosome 14. sc-142992-V. REFERENCES Molecular Weight of Dematin: 52/48 kDa. 1. Rana, A.P., et al. 1993. Cloning of human erythroid Dematin reveals Positive Controls: Hep G2 cell lysate: sc-2227. -

Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT) Beyond SMARCA4 Mutations: a Comprehensive Genomic Analysis
cells Article Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT) beyond SMARCA4 Mutations: A Comprehensive Genomic Analysis Aurélie Auguste 1,Félix Blanc-Durand 2 , Marc Deloger 3 , Audrey Le Formal 1, Rohan Bareja 4,5, David C. Wilkes 4 , Catherine Richon 6,Béatrice Brunn 2, Olivier Caron 6, Mojgan Devouassoux-Shisheboran 7,Sébastien Gouy 2, Philippe Morice 2, Enrica Bentivegna 2, Andrea Sboner 4,5,8, Olivier Elemento 4,8, Mark A. Rubin 9 , Patricia Pautier 2, Catherine Genestie 10, Joanna Cyrta 4,9,11 and Alexandra Leary 1,2,* 1 Medical Oncologist, Gynecology Unit, Lead Translational Research Team, INSERM U981, Gustave Roussy, 94805 Villejuif, France; [email protected] (A.A.); [email protected] (A.L.F.) 2 Gynecological Unit, Department of Medicine, Gustave Roussy, 94805 Villejuif, France; [email protected] (F.B.-D.); [email protected] (B.B.); [email protected] (S.G.); [email protected] (P.M.); [email protected] (E.B.); [email protected] (P.P.) 3 Bioinformatics Core Facility, Gustave Roussy Cancer Center, UMS CNRS 3655/INSERM 23 AMMICA, 94805 Villejuif, France; [email protected] 4 Caryl and Israel Englander Institute for Precision Medicine, Weill Cornell Medicine, New York, NY 10001, USA; [email protected] (R.B.); [email protected] (D.C.W.); [email protected] (A.S.); [email protected] (O.E.); [email protected] (J.C.) 5 Institute for Computational Biomedicine, Weill Cornell -

Aneuploidy: Using Genetic Instability to Preserve a Haploid Genome?
Health Science Campus FINAL APPROVAL OF DISSERTATION Doctor of Philosophy in Biomedical Science (Cancer Biology) Aneuploidy: Using genetic instability to preserve a haploid genome? Submitted by: Ramona Ramdath In partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biomedical Science Examination Committee Signature/Date Major Advisor: David Allison, M.D., Ph.D. Academic James Trempe, Ph.D. Advisory Committee: David Giovanucci, Ph.D. Randall Ruch, Ph.D. Ronald Mellgren, Ph.D. Senior Associate Dean College of Graduate Studies Michael S. Bisesi, Ph.D. Date of Defense: April 10, 2009 Aneuploidy: Using genetic instability to preserve a haploid genome? Ramona Ramdath University of Toledo, Health Science Campus 2009 Dedication I dedicate this dissertation to my grandfather who died of lung cancer two years ago, but who always instilled in us the value and importance of education. And to my mom and sister, both of whom have been pillars of support and stimulating conversations. To my sister, Rehanna, especially- I hope this inspires you to achieve all that you want to in life, academically and otherwise. ii Acknowledgements As we go through these academic journeys, there are so many along the way that make an impact not only on our work, but on our lives as well, and I would like to say a heartfelt thank you to all of those people: My Committee members- Dr. James Trempe, Dr. David Giovanucchi, Dr. Ronald Mellgren and Dr. Randall Ruch for their guidance, suggestions, support and confidence in me. My major advisor- Dr. David Allison, for his constructive criticism and positive reinforcement. -

Frequent Loss of Heterozygosity Targeting the Inactive X Chromosome in Melanoma
6476 Vol. 9, 6476–6482, December 15, 2003 Clinical Cancer Research Frequent Loss of Heterozygosity Targeting the Inactive X Chromosome in Melanoma James O. Indsto,1 Najah T. Nassif,2 INTRODUCTION Richard F. Kefford,1 and Graham J. Mann1 Chromosomal deletions and amplification events are fre- 1Westmead Institute for Cancer Research, University of Sydney at quent in advanced neoplasms and provide evidence for the Westmead Millennium Institute, Westmead, New South Wales, existence and location of putative tumor suppressor genes Australia, and 2Department of Cell and Molecular Biology, University (TSGs) and oncogenes. In melanoma, 9p and 10q deletions are of Technology, Sydney, New South Wales, Australia most frequent, and losses on 6q, 11q, 1p, 15p, 17q, and 18q are also frequently found (1, 2). However, no studies have focused on the X chromosome. In the human female, random inactiva- ABSTRACT tion of one copy of an X chromosome by hypermethylation is an After previous preliminary observations of paradoxical early event in embryogenesis, resulting in most tissues being a deletion events affecting the inactive X chromosome in mel- mosaic in terms of X inactivation status. Neoplasms, being anoma, we have surveyed the X chromosome for deletions clonal expansions of single precursor cells, usually share a using 23 polymorphic microsatellite markers in 28 inform- common X inactivation status. This can be conveniently assayed ative (female XX) metastatic melanomas. Ten tumors (36%) using the methylation-sensitive restriction enzyme HpaII, which showed at least one loss of heterozygosity (LOH) event, and has a recognition site within the highly polymorphic exon 1 in two cases an entire chromosome showed LOH at all CAG repeat (ARTR) in the androgen receptor (AR) gene. -

Microarray Analysis of Novel Genes Involved in HSV- 2 Infection
Microarray analysis of novel genes involved in HSV- 2 infection Hao Zhang Nanjing University of Chinese Medicine Tao Liu ( [email protected] ) Nanjing University of Chinese Medicine https://orcid.org/0000-0002-7654-2995 Research Article Keywords: HSV-2 infection,Microarray analysis,Histospecic gene expression Posted Date: May 12th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-517057/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/19 Abstract Background: Herpes simplex virus type 2 infects the body and becomes an incurable and recurring disease. The pathogenesis of HSV-2 infection is not completely clear. Methods: We analyze the GSE18527 dataset in the GEO database in this paper to obtain distinctively displayed genes(DDGs)in the total sequential RNA of the biopsies of normal and lesioned skin groups, healed skin and lesioned skin groups of genital herpes patients, respectively.The related data of 3 cases of normal skin group, 4 cases of lesioned group and 6 cases of healed group were analyzed.The histospecic gene analysis , functional enrichment and protein interaction network analysis of the differential genes were also performed, and the critical components were selected. Results: 40 up-regulated genes and 43 down-regulated genes were isolated by differential performance assay. Histospecic gene analysis of DDGs suggested that the most abundant system for gene expression was the skin, immune system and the nervous system.Through the construction of core gene combinations, protein interaction network analysis and selection of histospecic distribution genes, 17 associated genes were selected CXCL10,MX1,ISG15,IFIT1,IFIT3,IFIT2,OASL,ISG20,RSAD2,GBP1,IFI44L,DDX58,USP18,CXCL11,GBP5,GBP4 and CXCL9.The above genes are mainly located in the skin, immune system, nervous system and reproductive system.