Gene Expression Studies: from Case-Control to Multiple-Population-Based Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analysis of Trans Esnps Infers Regulatory Network Architecture
Analysis of trans eSNPs infers regulatory network architecture Anat Kreimer Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2014 © 2014 Anat Kreimer All rights reserved ABSTRACT Analysis of trans eSNPs infers regulatory network architecture Anat Kreimer eSNPs are genetic variants associated with transcript expression levels. The characteristics of such variants highlight their importance and present a unique opportunity for studying gene regulation. eSNPs affect most genes and their cell type specificity can shed light on different processes that are activated in each cell. They can identify functional variants by connecting SNPs that are implicated in disease to a molecular mechanism. Examining eSNPs that are associated with distal genes can provide insights regarding the inference of regulatory networks but also presents challenges due to the high statistical burden of multiple testing. Such association studies allow: simultaneous investigation of many gene expression phenotypes without assuming any prior knowledge and identification of unknown regulators of gene expression while uncovering directionality. This thesis will focus on such distal eSNPs to map regulatory interactions between different loci and expose the architecture of the regulatory network defined by such interactions. We develop novel computational approaches and apply them to genetics-genomics data in human. We go beyond pairwise interactions to define network motifs, including regulatory modules and bi-fan structures, showing them to be prevalent in real data and exposing distinct attributes of such arrangements. We project eSNP associations onto a protein-protein interaction network to expose topological properties of eSNPs and their targets and highlight different modes of distal regulation. -
SM08 Programme
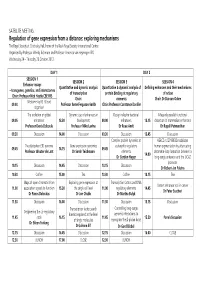
SATELLITE MEETING Regulation of gene expression from a distance: exploring mechanisms The Royal Society at Chicheley Hall, home of the Kavli Royal Society International Centre Organised by Professor Wendy Bickmore and Professor Veronica van Heyningen FRS Wednesday 24 – Thursday 25 October 2012 DAY 1 DAY 2 SESSION 1 SESSION 2 SESSION 3 SESSION 4 Enhancer assays Quantitative and dynamic analysis Quantitative & dynamic analysis of Defining enhancers and their mechanisms – transgenes, genetics, and interactomes of transcription protein binding at regulatory of action Chair: Professor Nick Hastie CBE FRS Chair: elements Chair: Dr Duncan Odom Welcome by RS & lead 09.00 Professor Anne Ferguson-Smith Chair: Professor Constance Bonifer organiser The evolution of global Dynamic use of enhancers in Design rules for bacterial Massively parallel functional 09.05 enhancers 13.30 development 09.00 enhancers 13.15 dissection of mammalian enhancers Professor Denis Duboule Professor Mike Levine Dr Roee Amit Dr Rupali Patwardhan 09.30 Discussion 14.00 Discussion 09.30 Discussion 13.45 Discussion Complex protein dynamics at HERC2 rs12913832 modulates The pluripotent 3D genome Gene expression genomics eukaryotic regulatory human pigmentation by attenuating 09.45 14.15 09.45 Professor Wouter de Laat Dr Sarah Teichmann elements chromatin-loop formation between a 14.00 Dr Gordon Hager long-range enhancer and the OCA2 promoter 10.15 Discussion 14.45 Discussion 10.15 Discussion Dr Robert-Jan Palstra 10.30 Coffee 15.00 Tea 10.30 Coffee 14.15 Tea Maps of open chromatin -

Identification of the Binding Partners for Hspb2 and Cryab Reveals
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2013-12-12 Identification of the Binding arP tners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactions and Non- Redundant Roles for Small Heat Shock Proteins Kelsey Murphey Langston Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Microbiology Commons BYU ScholarsArchive Citation Langston, Kelsey Murphey, "Identification of the Binding Partners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactions and Non-Redundant Roles for Small Heat Shock Proteins" (2013). Theses and Dissertations. 3822. https://scholarsarchive.byu.edu/etd/3822 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Identification of the Binding Partners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactions and Non-Redundant Roles for Small Heat Shock Proteins Kelsey Langston A thesis submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Master of Science Julianne H. Grose, Chair William R. McCleary Brian Poole Department of Microbiology and Molecular Biology Brigham Young University December 2013 Copyright © 2013 Kelsey Langston All Rights Reserved ABSTRACT Identification of the Binding Partners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactors and Non-Redundant Roles for Small Heat Shock Proteins Kelsey Langston Department of Microbiology and Molecular Biology, BYU Master of Science Small Heat Shock Proteins (sHSP) are molecular chaperones that play protective roles in cell survival and have been shown to possess chaperone activity. -

In Vivo Genome-Wide Binding Interactions of Mouse and Human Constitutive Androstane Receptors Reveal Novel Gene Targets Ben Niu1, Denise M
Published online 8 August 2018 Nucleic Acids Research, 2018, Vol. 46, No. 16 8385–8403 doi: 10.1093/nar/gky692 In vivo genome-wide binding interactions of mouse and human constitutive androstane receptors reveal novel gene targets Ben Niu1, Denise M. Coslo1, Alain R. Bataille2, Istvan Albert2, B. Franklin Pugh2 and Curtis J. Omiecinski1,* 1Center for Molecular Toxicology and Carcinogenesis, Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, University Park, PA 16802, USA and 2Department of Biochemistry and Molecular Biology, The Pennsylvania State University, University Park, PA 16802, USA Received May 04, 2018; Revised July 17, 2018; Editorial Decision July 19, 2018; Accepted July 20, 2018 ABSTRACT tion factors that are activated by direct ligands (1) and gen- erally share a common structure, an N-terminal A/B do- The constitutive androstane receptor (CAR; NR1I3) main, a DNA binding domain (DBD), a hinge domain, a is a nuclear receptor orchestrating complex roles ligand binding/ heterodimerization domain (LBD) and an in cell and systems biology. Species differences in F domain at the C-terminal (2). Upon ligand activation, CAR’s effector pathways remain poorly understood, the LBD would utilize activation function-2 (AF-2) to me- including its role in regulating liver tumor promotion. diate co-regulator interactions (3). However, CAR is dis- We developed transgenic mouse models to assess tinguished from other nuclear receptors in that it lacks an genome-wide binding of mouse and human CAR, A/B domain and is constitutively active in the absence of following receptor activation in liver with direct lig- ligand due to a charge-charge interaction between LBD he- ands and with phenobarbital, an indirect CAR acti- lixes that mimic an active AF-2 conformation, and therefore vator. -

Protein Identities in Evs Isolated from U87-MG GBM Cells As Determined by NG LC-MS/MS
Protein identities in EVs isolated from U87-MG GBM cells as determined by NG LC-MS/MS. No. Accession Description Σ Coverage Σ# Proteins Σ# Unique Peptides Σ# Peptides Σ# PSMs # AAs MW [kDa] calc. pI 1 A8MS94 Putative golgin subfamily A member 2-like protein 5 OS=Homo sapiens PE=5 SV=2 - [GG2L5_HUMAN] 100 1 1 7 88 110 12,03704523 5,681152344 2 P60660 Myosin light polypeptide 6 OS=Homo sapiens GN=MYL6 PE=1 SV=2 - [MYL6_HUMAN] 100 3 5 17 173 151 16,91913397 4,652832031 3 Q6ZYL4 General transcription factor IIH subunit 5 OS=Homo sapiens GN=GTF2H5 PE=1 SV=1 - [TF2H5_HUMAN] 98,59 1 1 4 13 71 8,048185945 4,652832031 4 P60709 Actin, cytoplasmic 1 OS=Homo sapiens GN=ACTB PE=1 SV=1 - [ACTB_HUMAN] 97,6 5 5 35 917 375 41,70973209 5,478027344 5 P13489 Ribonuclease inhibitor OS=Homo sapiens GN=RNH1 PE=1 SV=2 - [RINI_HUMAN] 96,75 1 12 37 173 461 49,94108966 4,817871094 6 P09382 Galectin-1 OS=Homo sapiens GN=LGALS1 PE=1 SV=2 - [LEG1_HUMAN] 96,3 1 7 14 283 135 14,70620005 5,503417969 7 P60174 Triosephosphate isomerase OS=Homo sapiens GN=TPI1 PE=1 SV=3 - [TPIS_HUMAN] 95,1 3 16 25 375 286 30,77169764 5,922363281 8 P04406 Glyceraldehyde-3-phosphate dehydrogenase OS=Homo sapiens GN=GAPDH PE=1 SV=3 - [G3P_HUMAN] 94,63 2 13 31 509 335 36,03039959 8,455566406 9 Q15185 Prostaglandin E synthase 3 OS=Homo sapiens GN=PTGES3 PE=1 SV=1 - [TEBP_HUMAN] 93,13 1 5 12 74 160 18,68541938 4,538574219 10 P09417 Dihydropteridine reductase OS=Homo sapiens GN=QDPR PE=1 SV=2 - [DHPR_HUMAN] 93,03 1 1 17 69 244 25,77302971 7,371582031 11 P01911 HLA class II histocompatibility antigen, -

Exploring Prostate Cancer Genome Reveals Simultaneous Losses of PTEN, FAS and PAPSS2 in Patients with PSA Recurrence After Radical Prostatectomy
Int. J. Mol. Sci. 2015, 16, 3856-3869; doi:10.3390/ijms16023856 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Exploring Prostate Cancer Genome Reveals Simultaneous Losses of PTEN, FAS and PAPSS2 in Patients with PSA Recurrence after Radical Prostatectomy Chinyere Ibeawuchi 1, Hartmut Schmidt 2, Reinhard Voss 3, Ulf Titze 4, Mahmoud Abbas 5, Joerg Neumann 6, Elke Eltze 7, Agnes Marije Hoogland 8, Guido Jenster 9, Burkhard Brandt 10 and Axel Semjonow 1,* 1 Prostate Center, Department of Urology, University Hospital Muenster, Albert-Schweitzer-Campus 1, Gebaeude 1A, Muenster D-48149, Germany; E-Mail: [email protected] 2 Center for Laboratory Medicine, University Hospital Muenster, Albert-Schweitzer-Campus 1, Gebaeude 1A, Muenster D-48149, Germany; E-Mail: [email protected] 3 Interdisciplinary Center for Clinical Research, University of Muenster, Albert-Schweitzer-Campus 1, Gebaeude D3, Domagkstrasse 3, Muenster D-48149, Germany; E-Mail: [email protected] 4 Pathology, Lippe Hospital Detmold, Röntgenstrasse 18, Detmold D-32756, Germany; E-Mail: [email protected] 5 Institute of Pathology, Mathias-Spital-Rheine, Frankenburg Street 31, Rheine D-48431, Germany; E-Mail: [email protected] 6 Institute of Pathology, Klinikum Osnabrueck, Am Finkenhuegel 1, Osnabrueck D-49076, Germany; E-Mail: [email protected] 7 Institute of Pathology, Saarbrücken-Rastpfuhl, Rheinstrasse 2, Saarbrücken D-66113, Germany; E-Mail: [email protected] 8 Department -

Noninvasive Sleep Monitoring in Large-Scale Screening of Knock-Out Mice
bioRxiv preprint doi: https://doi.org/10.1101/517680; this version posted January 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Noninvasive sleep monitoring in large-scale screening of knock-out mice reveals novel sleep-related genes Shreyas S. Joshi1*, Mansi Sethi1*, Martin Striz1, Neil Cole2, James M. Denegre2, Jennifer Ryan2, Michael E. Lhamon3, Anuj Agarwal3, Steve Murray2, Robert E. Braun2, David W. Fardo4, Vivek Kumar2, Kevin D. Donohue3,5, Sridhar Sunderam6, Elissa J. Chesler2, Karen L. Svenson2, Bruce F. O'Hara1,3 1Dept. of Biology, University of Kentucky, Lexington, KY 40506, USA, 2The Jackson Laboratory, Bar Harbor, ME 04609, USA, 3Signal solutions, LLC, Lexington, KY 40503, USA, 4Dept. of Biostatistics, University of Kentucky, Lexington, KY 40536, USA, 5Dept. of Electrical and Computer Engineering, University of Kentucky, Lexington, KY 40506, USA. 6Dept. of Biomedical Engineering, University of Kentucky, Lexington, KY 40506, USA. *These authors contributed equally Address for correspondence and proofs: Shreyas S. Joshi, Ph.D. Dept. of Biology University of Kentucky 675 Rose Street 101 Morgan Building Lexington, KY 40506 U.S.A. Phone: (859) 257-2805 FAX: (859) 257-1717 Email: [email protected] Running title: Sleep changes in knockout mice bioRxiv preprint doi: https://doi.org/10.1101/517680; this version posted January 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Supporting Information
Supporting Information Muro et al. 10.1073/pnas.0807813105 SI Text (Fig. S1) showed for 10 of the PAS variants a marked tendency 1. Algorithm for Automated Recognition of EST End Clusters. To to lie 10–30 nt upstream of EST ends, whereas no strong trend determine the coordinates of genomic EST alignments we used was seen for 3 additional variants. Based on this simple analysis the UCSC genome annotation (1), a regularly updated database we chose to accept only 10 of the variants as functional PAS which includes mouse and human EST sequences and their (Table S1), and only EST ends validated by a PAS 10–30 nt positions in the genome. The UCSC Golden Path version was upstream. Furthermore, we considered all EST ends validated by mm6 for mouse (equivalent to NCBI build 34, March 2005) and the same PAS and ending within 20 nt of one another to hg18 for human (NCBI Build 36.1, March 2006). The UCSC represent the same transcript end and to be clustered together, mapping database is based on alignments of ESTs to the as others have also proposed (6). corresponding genome using the BLAT program (2). Because of Next, we studied the distribution of individual EST ends the relatively low accuracy of EST sequencing and recent around our predicted rough ends (Fig. S2). The graph indicates genomic duplications (3), some ESTs can be aligned to more that in a range of Ϫ200 to ϩ150 nt around these rough ends the than 1 genomic position. To avoid possible misidentifications, frequency of EST ends is distinguishable from the surrounding ambiguously aligning ESTs were excluded from our analysis. -

EMBC Annual Report 2007
EMBO | EMBC annual report 2007 EUROPEAN MOLECULAR BIOLOGY ORGANIZATION | EUROPEAN MOLECULAR BIOLOGY CONFERENCE EMBO | EMBC table of contents introduction preface by Hermann Bujard, EMBO 4 preface by Tim Hunt and Christiane Nüsslein-Volhard, EMBO Council 6 preface by Marja Makarow and Isabella Beretta, EMBC 7 past & present timeline 10 brief history 11 EMBO | EMBC | EMBL aims 12 EMBO actions 2007 15 EMBC actions 2007 17 EMBO & EMBC programmes and activities fellowship programme 20 courses & workshops programme 21 young investigator programme 22 installation grants 23 science & society programme 24 electronic information programme 25 EMBO activities The EMBO Journal 28 EMBO reports 29 Molecular Systems Biology 30 journal subject categories 31 national science reviews 32 women in science 33 gold medal 34 award for communication in the life sciences 35 plenary lectures 36 communications 37 European Life Sciences Forum (ELSF) 38 ➔ 2 table of contents appendix EMBC delegates and advisers 42 EMBC scale of contributions 49 EMBO council members 2007 50 EMBO committee members & auditors 2007 51 EMBO council members 2008 52 EMBO committee members & auditors 2008 53 EMBO members elected in 2007 54 advisory editorial boards & senior editors 2007 64 long-term fellowship awards 2007 66 long-term fellowships: statistics 82 long-term fellowships 2007: geographical distribution 84 short-term fellowship awards 2007 86 short-term fellowships: statistics 104 short-term fellowships 2007: geographical distribution 106 young investigators 2007 108 installation -

Dematin (18): Sc-135881
SAN TA C RUZ BI OTEC HNOL OG Y, INC . Dematin (18): sc-135881 BACKGROUND APPLICATIONS Caldesmon, Filamin 1, Nebulin, Villin, Plastin, ADF, Gelsolin, Dematin and Dematin (18) is recommended for detection of Dematin of mouse, rat and Cofilin are differentially expressed Actin binding proteins. Dematin is a human origin by Western Blotting (starting dilution 1:200, dilution range bundling protein of the erythrocyte membrane skeleton. Dematin is localized 1:100-1:1000), immunoprecipitation [1-2 µg per 100-500 µg of total protein to the spectrin-Actin junctions and its Actin-bundling activity is abolished (1 ml of cell lysate)] and immunofluorescence (starting dilution 1:50, dilution upon phosphorylation by cAMP-dependent protein kinase. It may also play a range 1:50-1:500). role in the regulation of cell shape, implying a role in tumorigenesis. Dematin Suitable for use as control antibody for Dematin siRNA (h): sc-105286, is a trimeric protein containing two identical subunits and a larger subunit. Dematin siRNA (m): sc-142992, Dematin shRNA Plasmid (h): sc-105286-SH, It is localized to the heart, brain, lung, skeletal muscle and kidney. The Dematin shRNA Plasmid (m): sc-142992-SH, Dematin shRNA (h) Lentiviral Dematin gene is located on human chromosome 8p21, a region frequently Particles: sc-105286-V and Dematin shRNA (m) Lentiviral Particles: delet ed in prostate cancer, and mouse chromosome 14. sc-142992-V. REFERENCES Molecular Weight of Dematin: 52/48 kDa. 1. Rana, A.P., et al. 1993. Cloning of human erythroid Dematin reveals Positive Controls: Hep G2 cell lysate: sc-2227. -

Mouse Wdr37 Conditional Knockout Project (CRISPR/Cas9)
https://www.alphaknockout.com Mouse Wdr37 Conditional Knockout Project (CRISPR/Cas9) Objective: To create a Wdr37 conditional knockout Mouse model (C57BL/6J) by CRISPR/Cas-mediated genome engineering. Strategy summary: The Wdr37 gene (NCBI Reference Sequence: NM_001039388 ; Ensembl: ENSMUSG00000021147 ) is located on Mouse chromosome 13. 14 exons are identified, with the ATG start codon in exon 2 and the TAA stop codon in exon 14 (Transcript: ENSMUST00000054251). Exon 3 will be selected as conditional knockout region (cKO region). Deletion of this region should result in the loss of function of the Mouse Wdr37 gene. To engineer the targeting vector, homologous arms and cKO region will be generated by PCR using BAC clone RP23-90H23 as template. Cas9, gRNA and targeting vector will be co-injected into fertilized eggs for cKO Mouse production. The pups will be genotyped by PCR followed by sequencing analysis. Note: Exon 3 starts from about 9.34% of the coding region. The knockout of Exon 3 will result in frameshift of the gene. The size of intron 2 for 5'-loxP site insertion: 4159 bp, and the size of intron 3 for 3'-loxP site insertion: 2736 bp. The size of effective cKO region: ~597 bp. The cKO region does not have any other known gene. Page 1 of 8 https://www.alphaknockout.com Overview of the Targeting Strategy Wildtype allele gRNA region 5' gRNA region 3' 1 3 14 Targeting vector Targeted allele Constitutive KO allele (After Cre recombination) Legends Exon of mouse Wdr37 Homology arm cKO region loxP site Page 2 of 8 https://www.alphaknockout.com Overview of the Dot Plot Window size: 10 bp Forward Reverse Complement Sequence 12 Note: The sequence of homologous arms and cKO region is aligned with itself to determine if there are tandem repeats. -

Aneuploidy: Using Genetic Instability to Preserve a Haploid Genome?
Health Science Campus FINAL APPROVAL OF DISSERTATION Doctor of Philosophy in Biomedical Science (Cancer Biology) Aneuploidy: Using genetic instability to preserve a haploid genome? Submitted by: Ramona Ramdath In partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biomedical Science Examination Committee Signature/Date Major Advisor: David Allison, M.D., Ph.D. Academic James Trempe, Ph.D. Advisory Committee: David Giovanucci, Ph.D. Randall Ruch, Ph.D. Ronald Mellgren, Ph.D. Senior Associate Dean College of Graduate Studies Michael S. Bisesi, Ph.D. Date of Defense: April 10, 2009 Aneuploidy: Using genetic instability to preserve a haploid genome? Ramona Ramdath University of Toledo, Health Science Campus 2009 Dedication I dedicate this dissertation to my grandfather who died of lung cancer two years ago, but who always instilled in us the value and importance of education. And to my mom and sister, both of whom have been pillars of support and stimulating conversations. To my sister, Rehanna, especially- I hope this inspires you to achieve all that you want to in life, academically and otherwise. ii Acknowledgements As we go through these academic journeys, there are so many along the way that make an impact not only on our work, but on our lives as well, and I would like to say a heartfelt thank you to all of those people: My Committee members- Dr. James Trempe, Dr. David Giovanucchi, Dr. Ronald Mellgren and Dr. Randall Ruch for their guidance, suggestions, support and confidence in me. My major advisor- Dr. David Allison, for his constructive criticism and positive reinforcement.